 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Growth Factors for Cell Culture
To support ongoing cell therapy manufacturers and developers, ACROBiosystems has developed a wide array of high-quality cytokines for the in vitro culture of immune cells, stem cells, organoids, and various other cell types. We also offer Premium(Pre-GMP) and GMP grades for our cytokines to better meet the needs of various drug development stages and applications. All the cytokines are manufactured using a similar production process, therefore enabling a seamless transition between grades. As a result, we enable you to seamlessly transition between our products and accelerate your research and development.
| Premium(Pre-GMP) Grade | GMP Grade | |
|---|---|---|
| Application | Research and Development; Preclinical research, seamless transition into clinical phases | Designed to meet clinical phase requirements and bolster your IND application to various regulatory bodies. |
| Quality System | ISO 9001 /ISO 13485 Certified | ISO 9001 /ISO 13485 Certified (Development stage) GMP Quality Management System (Production stage) |
| Production | ISO certified facilities | GMP certified facilities |
| Transient or stable cell lines | Stable cell lines (Full inspection according to USP and ChP) | |
| Animal-origin free materials or BSE/TSE free | Animal-origin free materials or BSE/TSE free | |
| Pharmaceutical-grade materials | Pharmaceutical-grade materials | |
| Strict sterilization filtration | Strict sterilization filtration | |
| Class C+A room with manual aseptic filling (ISO5) | B+A Cleanroom aseptic filling | |
| No additional virus clearance steps | Additional specific virus removal processes | |
| Quality Control | Sterility / Mycoplasma testing | Sterility / Mycoplasma testing |
| Endotoxin control and detection | Endotoxin control and detection | |
| Validated key equipment and analytical instruments | Validated equipment /analytical instruments/analytical methods(Audit trail available) | |
| Residual DNA/HCP testing | Residual DNA/HCP testing | |
| Limited adventitious agent testing | Stricter standards for viral residues alongside animal safety evaluations (select products). | |
| Documentation | Common regulatory support | Comprehensive regulatory support files |
| DMF filing (Few products) | DMF filing (All products) |
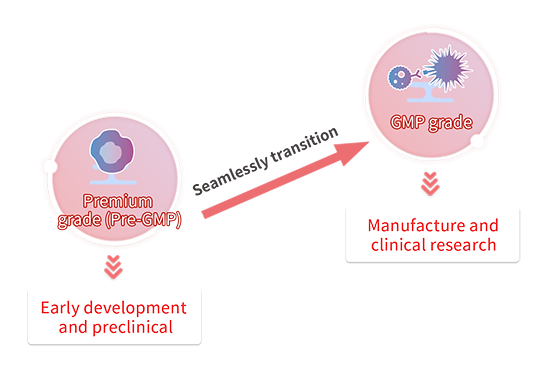
Transitioning from Premium(Pre-GMP) to GMP 
Cytokines are critical raw materials used in cell culturing for cell and gene therapy (CGT) drugs. Usually, in the preclinical stage, both safety and quality for raw materials are not prioritized. In this case, research-use only products can be used. However, when progressing into later drug development stages such as CMC or clinical phases, it is necessary to replace the raw materials with GMP-grade materials to be compliant with regulatory guidelines. In this transitional period, there is a significant amount of energy dedicated to re-evaluating and validating new raw materials.
To ease this transition from the preclinical development into the clinical stage, we offer several grades of cytokines that have been evaluated to be near-identical in bioactivity, along with the documentation required from GMP products. We recommend using our Premium(Pre-GMP) grade raw materials in the early development stage to seamlessly transition to our GMP-grade raw materials when entering CMC or clinical phases and minimize the number of re-evaluation and validation studies performed.
![]() Sterile
Sterile
![]() Animal-origin Free
Animal-origin Free
![]() High Purity≥95%
High Purity≥95%
![]() High Bioactivity Verified by Cell-based Assay
High Bioactivity Verified by Cell-based Assay
![]() Premium(Pre-GMP) and GMP Grades Available
Premium(Pre-GMP) and GMP Grades Available
![]() Low Endotoxin≤0.1 EU/μg
Low Endotoxin≤0.1 EU/μg
![]() Carrier Free
Carrier Free
![]() Similar to Natural Conformation and Modifications
Similar to Natural Conformation and Modifications
![]() Consistent between Batches
Consistent between Batches
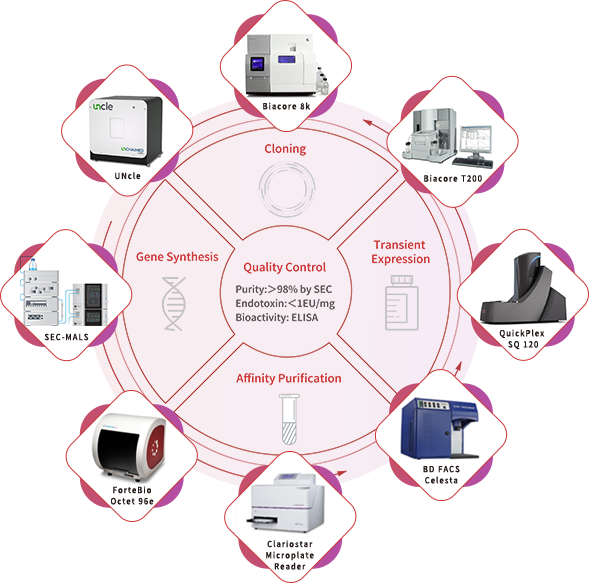
With a portfolio of over 5,000 recombinant proteins and an industry-leading, scale-up ready protein development platform, ACROBiosystems has accumulated over 10 years of experience in developing recombinant proteins. Using this platform, our custom GMP-grade protein services are designed to ensure that our proteins are both structurally designed and validated for cellular therapies manufacturing. We take care to adhere strictly to the GMP guidelines with our comprehensive quality management system and quality controls, providing you with high-quality raw materials without disrupting your development process. Our custom GMP-grade protein service is a one-stop service based on your needs to maximize your therapy’s success. We offer two different developmental processes: converting our non-GMP protein products to GMP or developing a custom GMP-grade protein product from scratch.
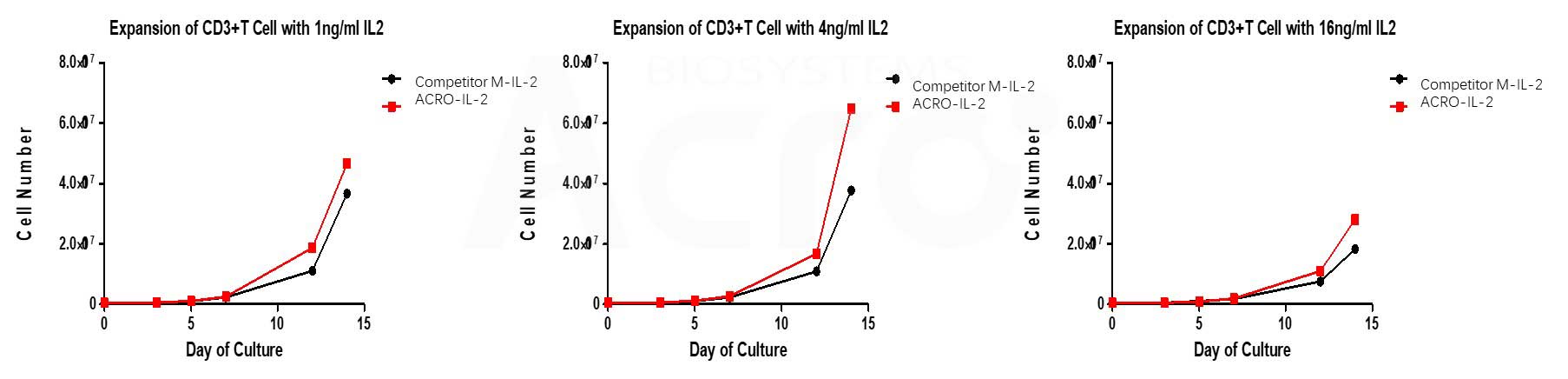
ACRO’s Human IL-2, premium grade (Cat. No.IL2-H5215) has higher bioactivity than that of other competitors when activates T cell with CD3/CD28 magnetic beads at different concentrations.
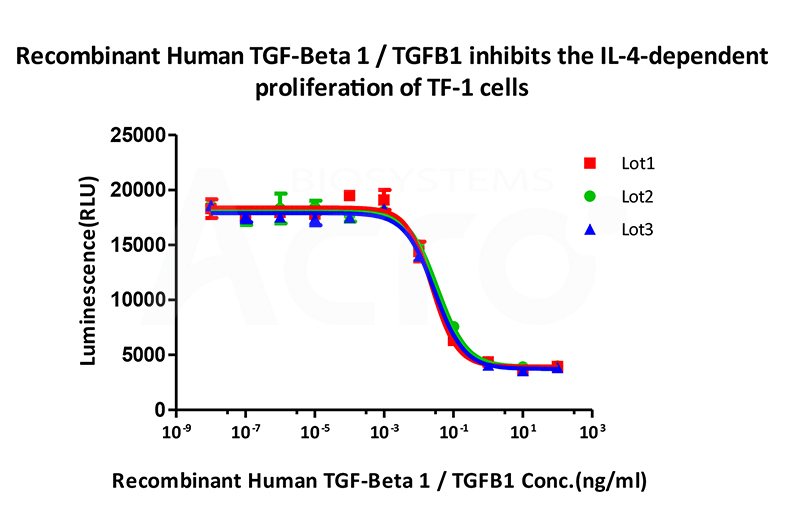
Bioactivity of three different lots of Recombinant Human TGF-Beta 1 TGFB1 (Cat. No. TG1-H4212) is verified by cell-based assay, and the result shows very high batch-to-batch consistency.
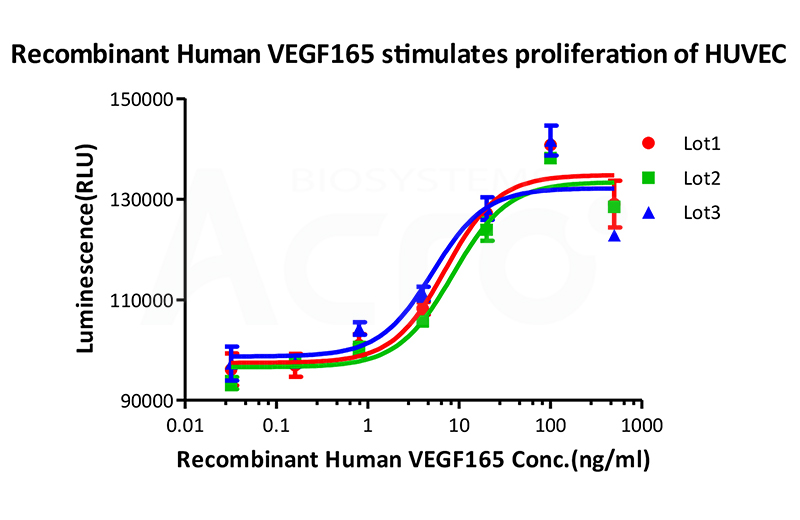
Bioactivity of three different lots of Recombinant Human VEGF165 (Cat. No. VE5-H4210) is verified by cell-based assay, and the result shows very high batch-to-batch consistency.
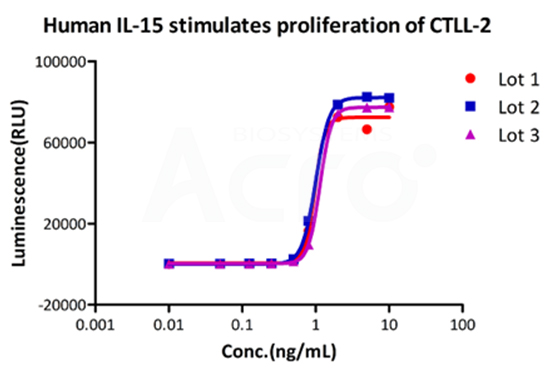
Recombinant Human IL-15 Protein (premium grade), designed for preclinical stage, has the same functional activity and performance as GMP Grade IL-15 (Cat. No.GMP-L15H13), which enables a seamless transition from preclinical development to clinical phases.
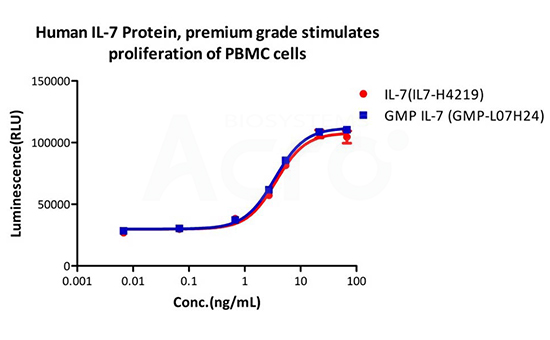
Human IL-7 Protein (premium grade) designed for preclinical stage, has the same functional activity and performance as GMP Grade IL-7 (Cat. No. GMP-L07H24), which enables a seamless transition from preclinical development to clinical phases.
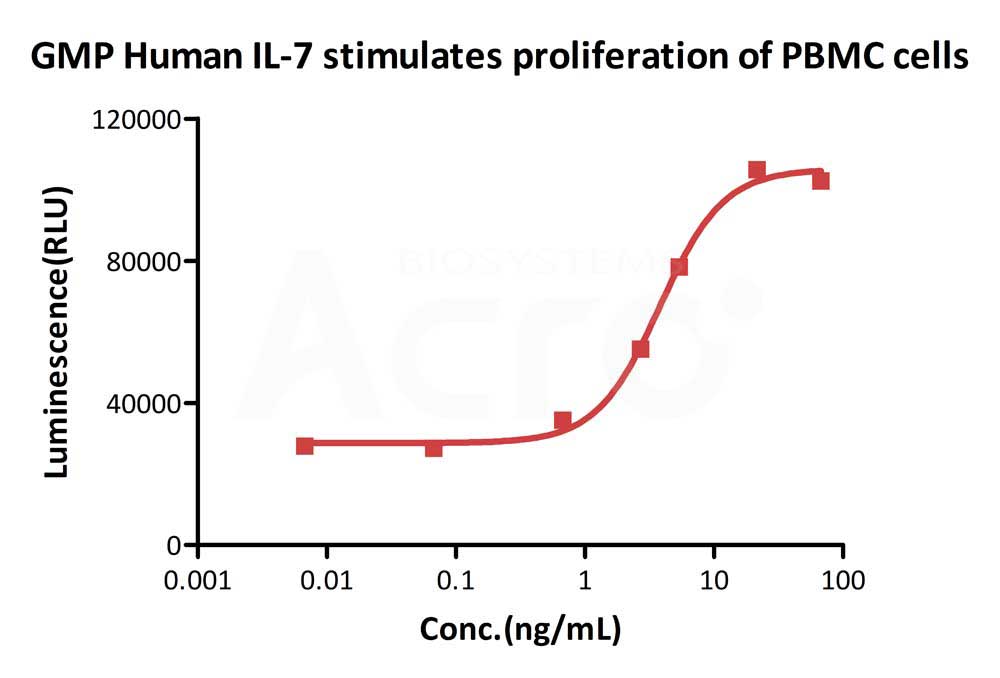
GMP Human IL-7 (Cat. No.GMP-L07H24) stimulates proliferation of PHA-P-activated human peripheral blood mononuclear cell (PBMC). The EC50 for this effect is 3.821 ng/mL, corresponding to a specific activity of > 1.0 ⅹ10^8 IU/mg, which is calibrated against human IL-7 WHO International Standard (NIBSC code: 90/530) (QC tested).
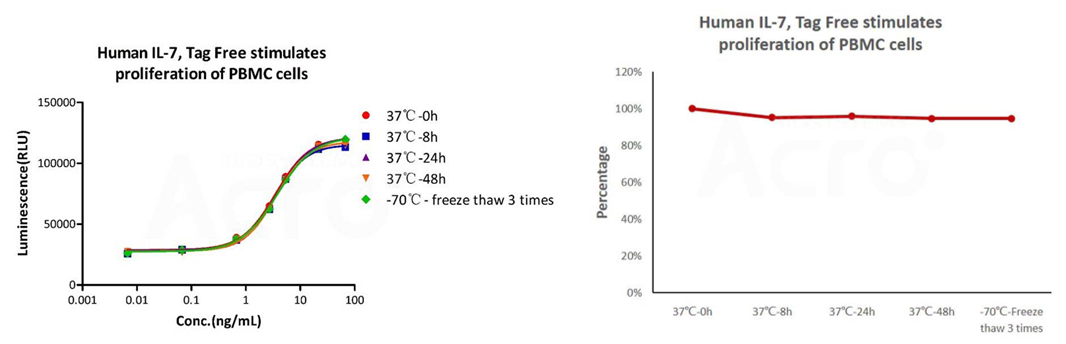
The cell-based assay shows that GMP Human IL-7 (Cat. No. GMP-L07H24) is stable at 37°C for 48 hours and after freezing and thawing 3 times.

Bioactivity of three different lots of GMP Human IL-15 (Cat. No.GMP-L15H13) verified by cell-based assay, and the result shows very high batch-to-batch consistency.
This web search service is supported by Google Inc.
