 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Kat. Nr. | Arten | Produktbeschreibung | Struktur | Reinheit | Merkmal |
|---|---|---|---|---|---|
| GMP-L02H14F002 | Human | GMP Human IL-2 Protein (Liquid) | 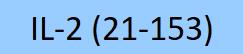 |
|
|
| RAS-SP006 | Mouse | Mouse IL-2 ELISPOT Kit | |||
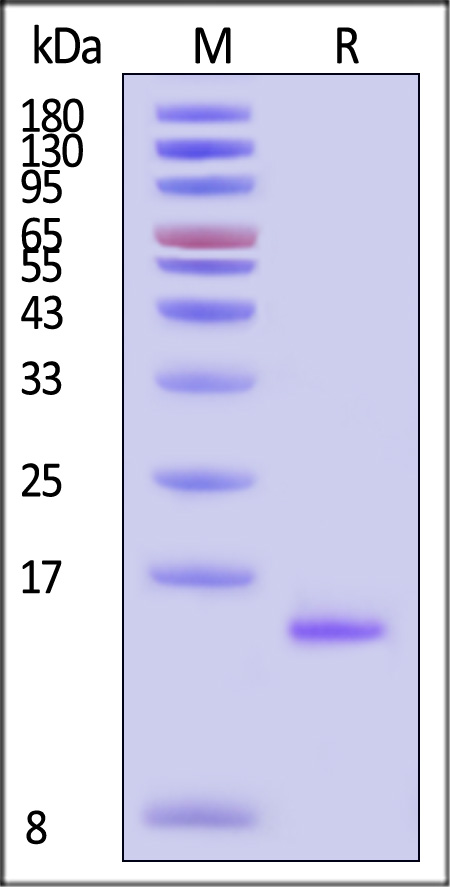
| GMP-L02H14 | Human | GMP Human IL-2 Protein |  |
|
|
| CRS-B008 | Human | ClinMax™ Human IL-2 ELISA Kit | |||
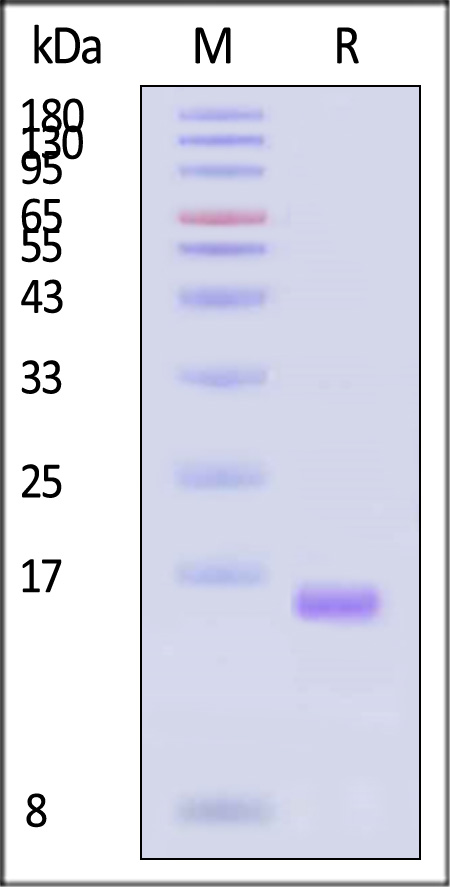
| IL2-H5215 | Human | Human IL-2 Protein, Tag Free (MALS verified) |  |
|
|
| CRS-A003 | Human | resDetect™ Human Interleukin-2 (IL-2) ELISA Kit (Residue Testing) | |||
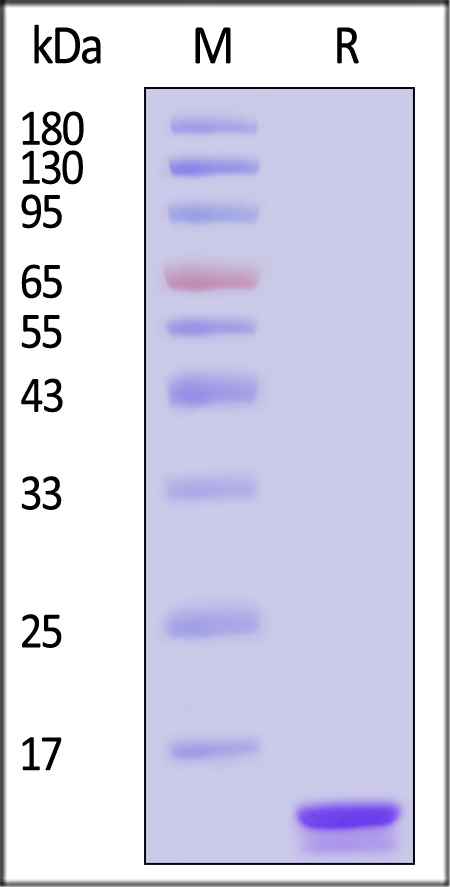
| IL2-C5249 | Cynomolgus | Cynomolgus IL-2 Protein, His Tag |  |

|
|
| IL2-H5269 | Human | Human IL-2 Protein, Fc Tag (MALS verified) |  |

|
|
| IL2-M52H3 | Mouse | Mouse IL-2 Protein, His Tag |  |

|
|
| IL2-H52H8 | Human | Human IL-2 Protein, His Tag |  |

|
|
| IL2-H82E4 | Human | Biotinylated Human IL-2 Protein, His,Avitag™ |  |

|
|
| IL2-H82F3 | Human | Biotinylated Human IL-2 Protein, Fc,Avitag™ |  |

|

GMP Human IL-2 Protein (Cat. No. GMP-L02H14) stimulates proliferation of CTLL-2 cells. The specific activity of GMP Human IL-2 Protein is ≥ 1.20×10^7 IU/mg, which is calibrated against human Interleukin-2 China National Standard (NIFDC code: 270008) (QC tested). China National Institutes for Food and Drug Control (NIFDC) Standard was prepared and calibrated against human IL-2 WHO International Standard (NIBSC code: 86/500) by NIFDC.
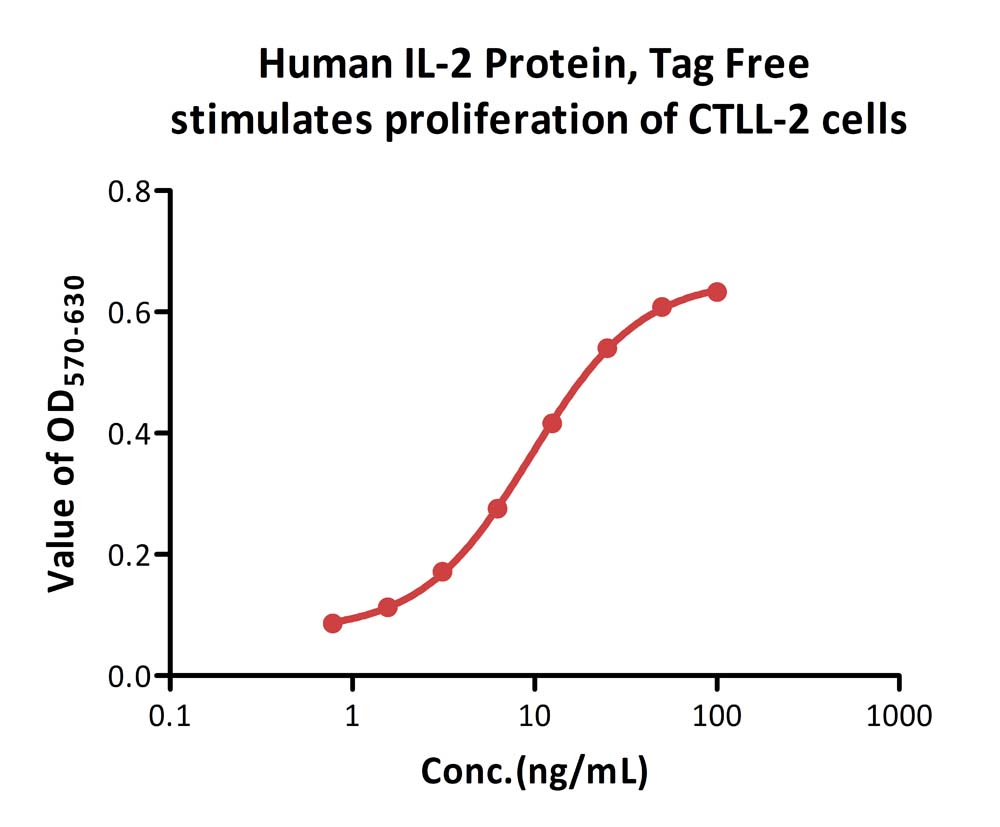
Human IL-2 Protein, Tag Free (Cat. No. IL2-H5215) stimulates proliferation of CTLL-2 cells. The specific activity of Human IL-2 Protein, Tag Free is > 5.00×10^6 IU/mg, which is calibrated against Interleukin-2 (Human, rDNA derived) (2nd International Standard) (NIBSC code: 86/500) (QC tested).

Cynomolgus IL-2 R beta, His Tag (Cat. No. ILB-C52H9) immobilized on CM5 Chip can bind Cynomolgus IL-2, His Tag (Cat. No. IL2-C5249) with an affinity constant of 377 nM as determined in a SPR assay (Biacore 8K) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Thalidomide | NSC-66847; NSC-527179; K-17; VP-02; FPF-300; FPF300 | Approved | Celgene Corp | Talizer, Thalidomide Celgene, Thalidomide Pharmion, Synovir, Thalomid, Thaled | Mainland China | Leprosy, Lepromatous; Multiple Myeloma | Changzhou Pharmaceutical Factory | 1982-01-01 | HIV Wasting Syndrome; Angiodysplasia; Primary Myelofibrosis; Neuroectodermal Tumors, Primitive; Prostatitis; Colorectal Neoplasms; Osteosarcoma; Lymphoma, Mantle-Cell; Sarcoma, Ewing; Retinoblastoma; Erythema Nodosum; Drug Resistant Epilepsy; Xerostomia; Sarcoma; Pancreatitis, Chronic; Adenocarcinoma, Clear Cell; Lymphoma, Follicular; Arachnoiditis; Carcinoma, Adenosquamous; Gastrointestinal Hemorrhage; Cholangitis, Sclerosing; Prostatic Neoplasms; Pelvic Pain; Neoplasm Metastasis; Stomatitis; Burning Mouth Syndrome; Mycobacterium avium-intracellulare Infection; Amyotrophic Lateral Sclerosis; Melanoma; Myelodysplastic-Myeloproliferative Diseases; Carcinoma, Hepatocellular; Leukemia, Lymphocytic, Chronic, B-Cell; Vascular Malformations; Tuberculosis; Appendiceal Neoplasms; Lymphoma, Non-Hodgkin; Uterine Neoplasms; Anemia, Sideroblastic; Glioma; Leprosy, Lepromatous; Endometrial Neoplasms; Lung Neoplasms; Waldenstrom Macroglobulinemia; Kidney Neoplasms; Thalassemia; Carcinoid Tumor; Lupus Erythematosus, Discoid | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Darleukin/fibromun | L19-IL-2/ L19-TNF-α | Phase 3 Clinical | Philogen Spa | Carcinoma, Merkel Cell; Carcinoma, Basal Cell; Carcinoma, Skin Appendage; Keratoacanthoma; Basal Cell Nevus Syndrome; Carcinoma, Squamous Cell; Lymphoma, T-Cell, Cutaneous; Melanoma; Sarcoma, Kaposi | Details |
| Bifikafusp alfa | L19-IL-2 | Phase 3 Clinical | Philogen Spa | Solid tumours; Melanoma; Carcinoma, Non-Small-Cell Lung; Neoplasm Metastasis | Details |
| BNZ-1 | BNZ132-1-40; BNZ-1; BNZ132-1; BNZ-01; EQ101; EQ-101 | Phase 2 Clinical | Bioniz Therapeutics Inc | Alopecia; Alopecia Areata; Lymphoma, T-Cell, Cutaneous; Leukemia, Large Granular Lymphocytic | Details |
| umitrelimorgene autodencel | ITI-1000 | Phase 2 Clinical | Immunomic Therapeutics Inc | Glioblastoma | Details |
| S-95007 | S-95007 | Phase 2 Clinical | Laboratoires Servier | Immune System Diseases; Inflammation | Details |
| AU-007 | BD-8; AU-007; BDG-8 | Phase 2 Clinical | Biolojic Design Inc | Solid tumours; Neoplasms; Neoplasm Metastasis | Details |
| XTX-202 | XTX-202 | Phase 2 Clinical | Xilio Development Inc, Xilio Therapeutics Inc | Solid tumours; Carcinoma, Renal Cell; Melanoma | Details |
| LAT-010 | LAT010; LAT-010 | Phase 2 Clinical | Latticon Antibody Therapeutics Inc | Solid tumours | Details |
| COYA-302 | COYA302; COYA 302; COYA-302 | Phase 2 Clinical | Coya Therapeutics Inc | Parkinson Disease; Frontotemporal Dementia; Amyotrophic Lateral Sclerosis | Details |
| AVB-001 | AVB-001 | Phase 2 Clinical | Avenge Bio Inc | Ovarian Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms | Details |
| Saltikva | Phase 2 Clinical | Salspera LLC | Pancreatic Neoplasms | Details | |
| LMBP2-specific TCR-T cell therapy (TCRCure Biopharma) | Phase 2 Clinical | Tcrcure Biopharma Ltd | Nasopharyngeal Carcinoma | Details | |
| Interleukin-2 gene therapy (St Jude Children's Research Hospital) | Phase 1 Clinical | St Jude Children's Research Hospital, National Cancer Institute | Neuroblastoma | Details | |
| T20K | T-20K | Phase 1 Clinical | Cyxone | Multiple Sclerosis | Details |
| SIM-0278 | ALM-223; SIM0278 | Phase 1 Clinical | Simcere Pharmaceutical Group Ltd | Autoimmune Diseases; Lupus Erythematosus, Systemic; Dermatitis, Atopic | Details |
| Mableukin 2PD1 (Anwita Biosciences) | AWT020; AWT-020 | Phase 1 Clinical | Anwita Biosciences Inc | Neoplasms; Autoimmune Diseases of the Nervous System | Details |
| AB-248 | AB-248 | Phase 1 Clinical | Asher Biotherapeutics Inc | Solid tumours; Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Melanoma; Carcinoma, Non-Small-Cell Lung | Details |
| ASP-1012 | VET2-L2; ASP-1012; ASP1012 | Phase 1 Clinical | KaliVir Immunotherapeutics Inc | Solid tumours | Details |
| STK-012 | STK-012 | Phase 1 Clinical | Synthekine Inc | Solid tumours; Mismatch Repair Deficient Cancer; Stomach Neoplasms; Squamous Cell Carcinoma of Head and Neck; Esophageal Neoplasms; Carcinoma, Renal Cell; Carcinoma, Transitional Cell; Microsatellite instability-high cancer; Uterine Cervical Neoplasms; Melanoma; Carcinoma, Non-Small-Cell Lung | Details |
| IL2 CD25 fusion protein (Bristol-Myers Squibb) | Phase 1 Clinical | University Of Miami | Autoimmune Diseases | Details | |
| M5A-IL2 Immunocytokine | M5A-ICK | Phase 1 Clinical | National Cancer Institute, City Of Hope National Medical Center | Breast Neoplasms; Colorectal Neoplasms | Details |
| DF-6215 | DF6215; DF-6215 | Phase 1 Clinical | Dragonfly Therapeutics Llc | Solid tumours | Details |
| Dodekin | Phase 1 Clinical | Philogen Spa | Solid tumours; Neoplasm Metastasis | Details | |
| KB-707 | KB-707; KB707 | Phase 1 Clinical | Krystal Biotech Inc | Solid tumours; Skin Melanoma; Carcinoma, Basal Cell; Carcinoma, Renal Cell; Small Cell Lung Carcinoma; Neoplasms; Sarcoma; Osteosarcoma; Colorectal Neoplasms; Carcinoma, Squamous Cell; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung; Melanoma; Carcinoma, Hepatocellular | Details |
| attIL2-T cell therapy | Phase 1 Clinical | The University Of Texas MD Anderson Cancer Center | Soft Tissue Neoplasms; Osteosarcoma; Sarcoma | Details | |
| Autologous tumor-infiltrating lymphocyte cells therapy (Hervor Therapeutics) | HV-101; HV101 | Phase 1 Clinical | Hervor Therapeutics, Tcrcure Biopharma Ltd, Hangzhou Houwu Biomedical Technology Co Ltd | Solid tumours | Details |
| KY-0118 | KY-0118; KY0118 | Phase 1 Clinical | Novatim Immune Therapeutics (Zhejiang) Co Ltd | Solid tumours; Carcinoma, Renal Cell; Carcinoma, Transitional Cell; Neoplasms; Pancreatic Neoplasms; Colorectal Neoplasms; Melanoma; Carcinoma, Non-Small-Cell Lung | Details |
| VIS171 | VIS-171; VIS171 | Phase 1 Clinical | Visterra Inc | Autoimmune Diseases | Details |
| MK-1484 | MK-1484; SP-482 | Phase 1 Clinical | Merck & Co Inc, Sutro Biopharma Inc | Solid tumours | Details |
| SAR-444336 | SAR-444336 | Phase 1 Clinical | Sanofi | Inflammation | Details |
| RS-2102 | RS2102 | Phase 1 Clinical | Reistone Biopharma Co Ltd | Dermatitis, Atopic | Details |
| RG-6279 | RG-6279 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Solid tumours | Details |
| BNT-153 | BNT-153 | Phase 1 Clinical | Biontech Se | Solid tumours | Details |
| Igrelimogene litadenorepvec | PM-1016; PM1016; TILT-123 | Phase 1 Clinical | Tilt Biotherapeutics Ltd | Solid tumours; Ovarian Neoplasms; Squamous Cell Carcinoma of Head and Neck; Small Cell Lung Carcinoma; Peritoneal Neoplasms; Lung Neoplasms; Fallopian Tube Neoplasms; Melanoma; Carcinoma, Hepatocellular | Details |
This web search service is supported by Google Inc.
