 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
The kit is developed for quantitative detection of natural and recombinant human FGF basic/FGF2/bFGF in serum, plasma and cell culture supernatants.
It is for research use only.
| Analyte | bFGF/FGF2 |
| Assay Type | Sandwich-ELISA |
| Reactivity | Human |
| Sensitivity | 7 pg/ml |
| Range | 10.94 pg/mL-700 pg/mL |
| Assay Time | 2.75 hr |
| Sample Type | Cell Culture Supernatants, Plasma, Serum. |
| Sample volume | 50 uL |
| Format | 96-wells plate breakable into 12 x 8 wells strips |
Elevate your research experience with our Cytokine/Biomarker Detection Kits, where accuracy, reliability, and ease of use are converging to deliver exceptional results.
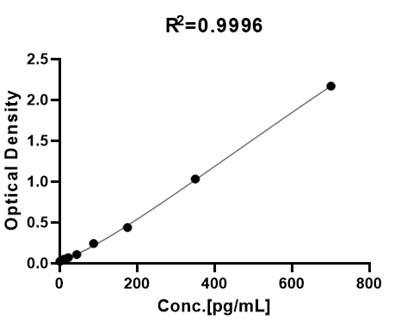
For each experiment, a standard curve needs to be set for each microplate, and the specific OD value may vary depending on different laboratories, testers, or equipment. The following example data is for reference only. The sample concentration was calculated based on the results of the standard curve. The minimum detectable concentration of bFGF/FGF2 is less than 7 pg/mL.
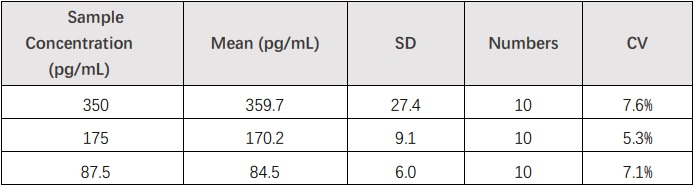
Ten replicates of each of 3 samples containing different bFGF/FGF2 concentrations were tested in one assay. Acceptable criteria: CV<10%.
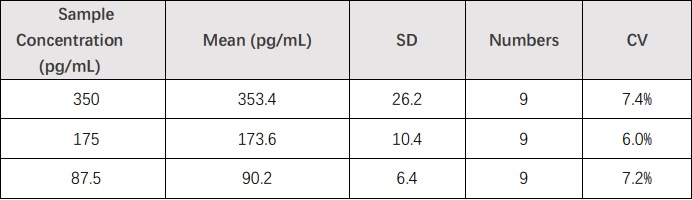
3 samples containing different concentrations of bFGF/FGF2 were tested in independent assays. Acceptable criteria: CV<15%.
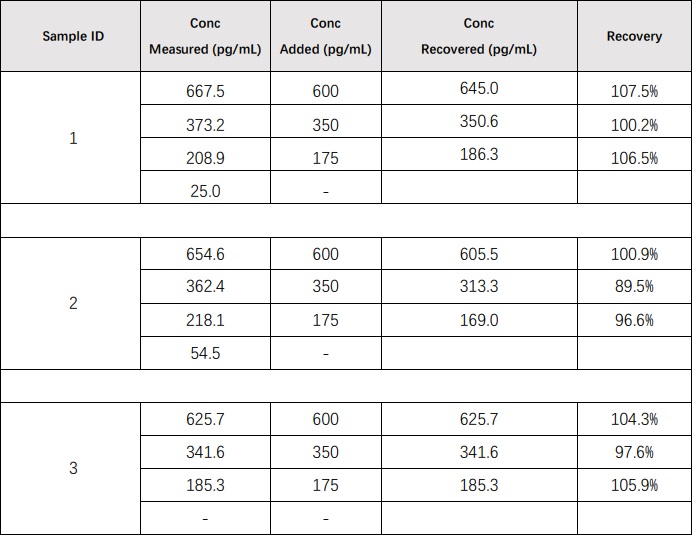
Recombinant bFGF/FGF2 was spiked into 3 human serum samples, and then analyzed. The average recovery of bFGF/FGF2 for serum samples is 100.9%.
| ID | Components | Size |
| CEA082-C01 | Pre-coated Anti-bFGF/FGF2 Antibody Microplate | 1 plate |
| CEA082-C02 | Human bFGF/FGF2 Standard | 20μg ×2 |
| CEA082-C03 | Biotin-Anti-bFGF/FGF2 Antibody Con. Solution | 100 μL |
| CEA082-C04 | Biotin-Antibody Dilution Buffer | 8 mL |
| CEA082-C05 | Streptavidin-HRP Con. Solution | 500 uL |
| CEA082-C06 | Streptavidin-HRP Dilution Buffer | 15 mL |
| CEA082-C07 | 20× Washing Buffer | 50 mL |
| CEA082-C08 | Sample Dilution Buffer | 15 mL ×2 |
| CEA082-C09 | Substrate Solution | 12 mL |
| CEA082-C10 | Stop Solution | 6 mL |
Price(EUR) : €500.00

Explore our catalog of therapeutic antibody solutions to find the right products for you! We are dedicated to delivering solutions designed to help you drive innovation and push the boundaries of what therapeutic antibodies can be.

We offer a wide range of cell and gene therapy solutions starting from discovery to the clinic. Explore our wide range of proteins, antibodies, kits, and other assays to accelerate the development of your cell and gene therapy.

Organoid Toolbox is a collection of organoid solutions including ready-to-use organoids, organoid differentiation kits, and a variety of services to accelerate the progress of your drug development project.

ACROBiosystems developed a series of GMP grade cytokines under the GMP grade quality management system. Those products are all suitable for T/NK cell generation, activation, and proliferation in cell therapy research.

50+ targets designed for CAR detection, including PE/FITC/biotin labeled proteins. The key reagents for CD19 and BCMA were FDA DMF filed which can support your IND, NDA and BLA process.

Full length multi-pass TPs with stabilized structure and high bioactivity for immunization, antibody screening, cell based assay and CAR detection, including hot CD20, Claudin 18.2, CD133, GPRC5D,CCR8, CCR5, etc.

GMP grade cytokines, reagents for cell activation, gene edition, DNA/RNA removal, etc. Particularly focus on product design, quality control and solution-based support to link each phase of your cell and gene therapy journey.

A series of immune checkpoints including classic co-inhibitory and co-stimulatory receptors. The comprehensive catalog contains 100+ targets with various species and tags, and the high-quality proteins are in good batch-to-batch consistency.

To meet the needs of ADCs development, ACROBiosystems can provide: A variety of high-quality target proteins; MMPs/Cathepsin/uPA for cleavable linker; Anti-payload antibodies & anti-idiotypic antibodies for immunogenicity and PK analysis; SPR/BLI analytical and ADA development service.

Comprehensive collection of Fc receptor proteins, including their common variants, which can help expedite your antibody development.

Comprehensive cytokine targets including interleukins, growth factors, chemokines, TNFs, etc. are expressed by HEK293 to ensure their natural structure. Their high purity is verified by SDS-PAGE/HPLC/SEC-MALS and high bioactivity is verified by ELISA/SPR/BLI.

Aneuro provides innovative solutions for neuroscience research. Recombinant proteins, neural factors, pre-formed fibrils, electrophysiological electrodes, as well as Organoid Toolbox all in Aneuro aiming to advance neuroscience research, develop therapeutic interventions, and improve diagnostic methods for neurological diseases.
This web search service is supported by Google Inc.
