 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
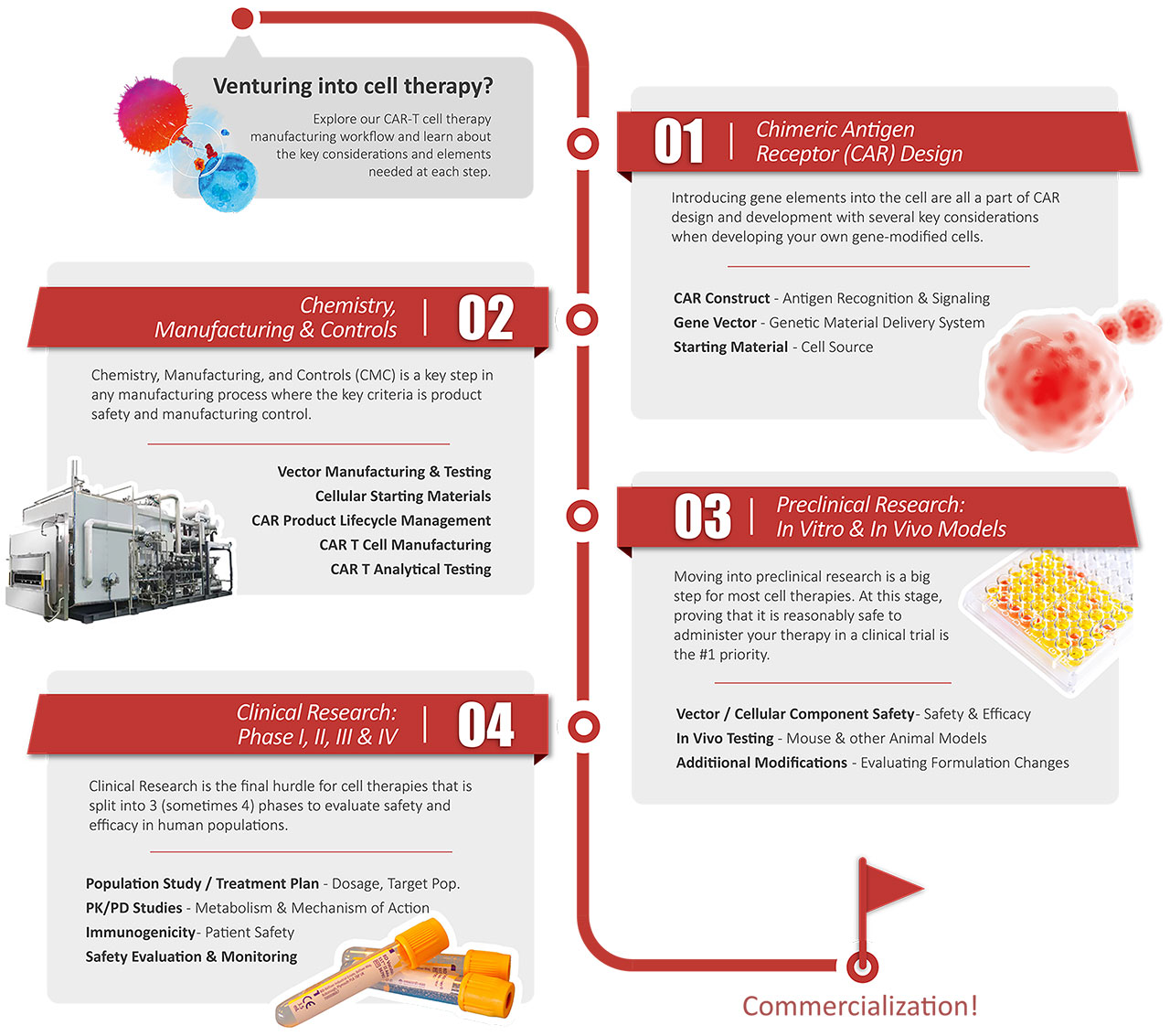
> Discovery Solutions for Cell and Gene Therapy
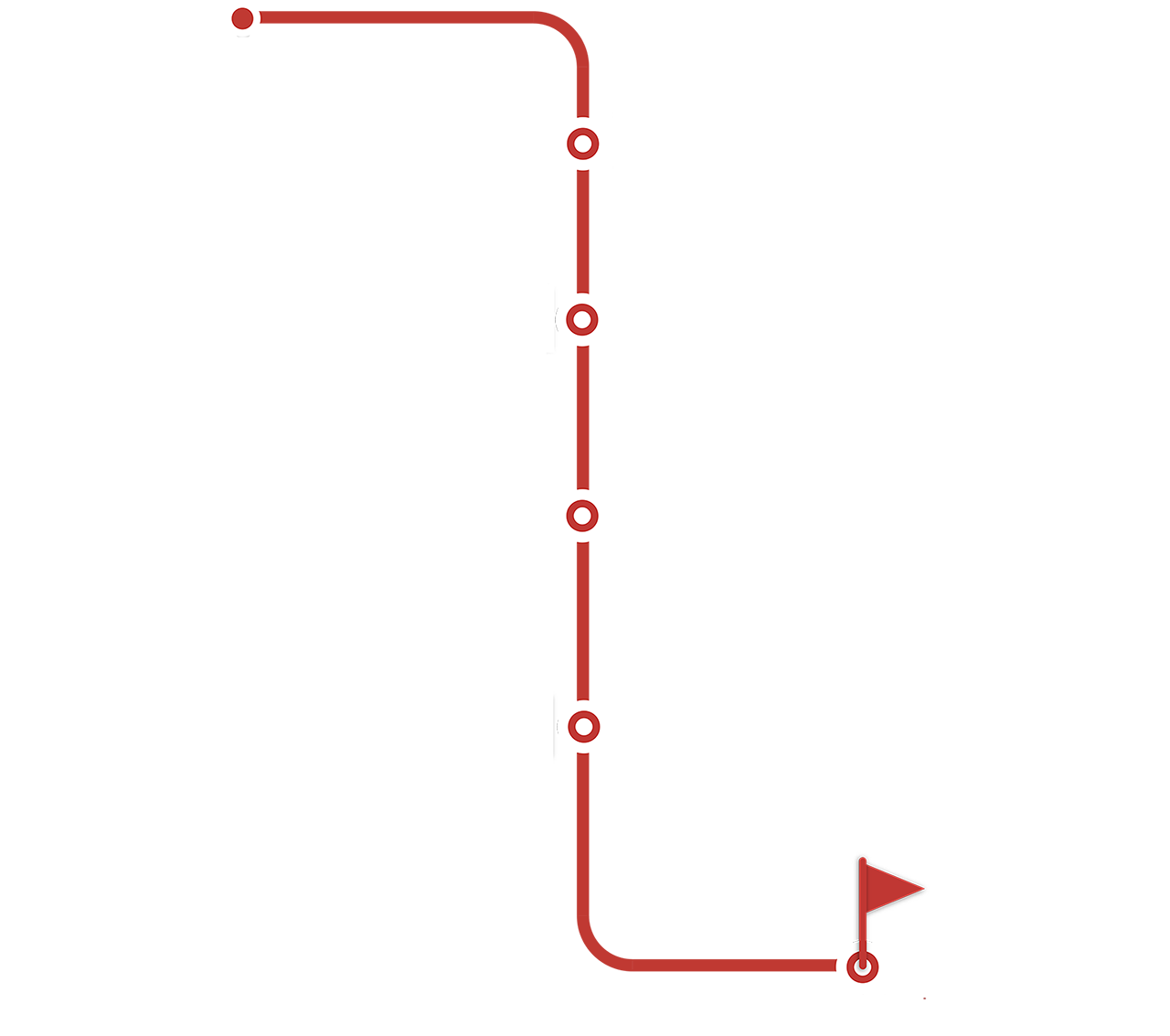
Explore our CAR-T cell therapy
manufacturing workflow and learn about
the key considerations and elements
needed at each step.
01
Chimeric Antigen
Receptor (CAR) Design
Introducing gene elements into the cell are all a part of CAR design and development with several key considerations when developing your own gene-modified cells.
CAR Construct - Antigen Recognition & Signaling
Gene Vector - Genetic Material Delivery System
Starting Material - Cell Source
Chemistry,
Manufacturing & Controls
02
Chemistry, Manufacturing. and Controls (CMC) is a key step in any manufacturing process where the key criteria is product safety and manufacturing control.
Vector Manufacturing & Testing
Cellular Starting Materials
CAR Product Lifecycle Management
CAR T Cell Manufacturing
CAR T Analytical Testing

03
Preclinical Research:
In Vitro & In Vivo Models
Moving into preclinical research is a big step for most cell therapies. At this stage, proving that it is reasonably safe to administer your therapy in a clinical trial is the #1 priority.
Vector / Cellular Component Safety - Safety & Efficacy
In Vivo Testing - Mouse & other Animal Models
Additional Modifications - Evaluating Formulation Changes
Clinical Research:
Phase I, Il, IIl & V
04
Clinical Research is the final hurdle for cell therapies that is split into 3 (sometimes 4) phases to evaluate safety and efficacy in human populations
Population Study / Treatment Plan - Dosage, Target Pop.
PK/PD Studies - Metabolism & Mechanism of Action
Immunogenicity - Patient Safety
Safety Evaluation & Monitoring
Cell therapies are a new, revolutionary type of medicine that has the potential to be a major breakthrough in advancing global health. No matter the type of cell therapy you are looking to discover, develop, manufacture, or commercialize, we offer an expansive catalog to help you throughout your cell and gene therapy drug development. Take advantage of our extensive portfolio and find the right solutions for you.
Custom GMP-grade Protein Services
Since cell therapies are personalized, shouldn’t the raw materials that you use to manufacture cell therapies be personal too? At ACROBiosystems, we offer custom GMP-grade protein development services that maintain the same, high-quality (and GMP-grade) that you expect from our products.


Cytokines & Growth Factors
RUO, Premium, GMP grade Materials
Cell culturing is an integral part of cell therapies, especially manufacturing. Whatever your needs are, from discovery to commercialization, we offer high-quality, validated cytokines and growth factors so you can have consistent results each and every time.
Establishing a GMP-grade Quality Management System
Especially regarding cell therapies, the safety and quality of raw materials is of the utmost importance. To comply with both domestic and international regulations, our quality management system implements stricter quality controls and process management to ensure that the products that you use are safe.


Residue Detection Kits
Ensuring the Safety of your Therapies
Since cell therapies are biological in origin, there are a significant amount of concerns with regards to the introduction of cross-species DNA and other genomic material. We offer a wide variety of nucleases to eliminate DNA/RNA traces, as well as kits to detect a wide variety of contaminants to ensure that your therapies comply with safety regulations.
This web search service is supported by Google Inc.
