 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Kat. Nr. | Arten | Produktbeschreibung | Struktur | Reinheit | Merkmal |
|---|---|---|---|---|---|
| PDA-H52H7 | Human | Human PDGF R alpha / PDGFRA Protein, His Tag | 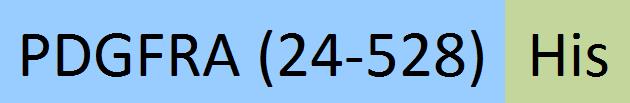 |
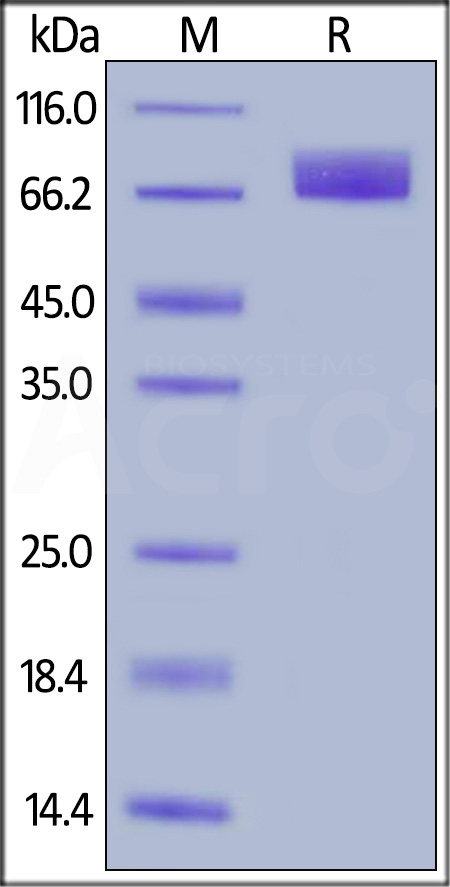
|

Human PDGF R alpha, His Tag (Cat. No. PDA-H52H7) immobilized on CM5 Chip can bind HHV-5 (strain AD169) gH&gL&gO, Twin-Strep Tag&Tag Free&His Tag with an affinity constant of 0.155 nM as determined in a SPR assay (Biacore 8K) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Lenvatinib Mesylate | MK-7902; ER-203492-00; E-7080 | Approved | Eisai Co Ltd | Kisplyx, Lenvima, Lenvima/Kisplyx, 乐卫玛 | United States | Thyroid Neoplasms | Eisai Inc | 2015-02-13 | Carcinoma, Adenoid Cystic; Paraganglioma; Melanoma; Thyroid Cancer, Papillary; Carcinoma, Hepatocellular; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Glioma; Lymphoma; Endometrial Neoplasms; Thyroid Neoplasms; Hepatic Insufficiency; Breast Neoplasms; Cholangiocarcinoma; Osteosarcoma; Solid tumours; Neuroendocrine Tumors; Adenocarcinoma, Follicular; Liver Diseases; Thyroid Carcinoma, Anaplastic; Adenocarcinoma of Lung; Kidney Diseases; Neoplasms; Pheochromocytoma; Esophageal Neoplasms; Renal Insufficiency; Carcinoma, Renal Cell; Liver Neoplasms; Ovarian Neoplasms; Biliary Tract Neoplasms | Details |
| Regorafenib | DAST; BAY-73-4506 | Approved | Bayer AG | Stivarga, Resihance | United States | Colorectal Neoplasms | Bayer Healthcare Pharmaceuticals Inc | 2012-09-27 | Fallopian Tube Neoplasms; Osteosarcoma; Sarcoma, Ewing; Colorectal Neoplasms; Gastrointestinal Stromal Tumors; Peritoneal Neoplasms; Bone Neoplasms; Bile Duct Neoplasms; Thymoma; Thyroid Neoplasms; Leukemia, Myeloid, Acute; Gastrinoma; Lung Neoplasms; Esophageal adenocarcinoma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Melanoma; Gastrointestinal Neoplasms; Somatostatinoma; Adenocarcinoma; Neoplasm Metastasis; Meningioma; Neoplasms; Solid tumours; Ovarian Neoplasms; Rectal Neoplasms; Carcinoma, Renal Cell; Hemangiosarcoma; Carcinoid Tumor; Insulinoma; Carcinoma, Islet Cell; Stomach Neoplasms; Esophageal Neoplasms; Liver Neoplasms; Carcinoma, Transitional Cell; Colonic Neoplasms; Pancreatic Neoplasms; Glioblastoma; Carcinoma, Ovarian Epithelial; Adenoma; Glucagonoma; Carcinoma, Adenoid Cystic; Sarcoma | Details |
| Olaratumab | IMC-3G3; LY-3012207 | Approved | Eli Lilly And Company | Lartruvo | United States | Sarcoma | Eli Lilly And Company | 2016-10-19 | Solid tumours; Ovarian Neoplasms; Fibrosarcoma; Leiomyosarcoma; Hemangiosarcoma; Dermatofibrosarcoma; Neoplasms; Sarcoma, Synovial; Liposarcoma; Sarcoma; Prostatic Neoplasms; Neurofibrosarcoma; Gastrointestinal Stromal Tumors; Carcinoma, Non-Small-Cell Lung; Neoplasm Metastasis | Details |
| Nintedanib Esylate | BIBF-1120 | Approved | C.H. Boehringer Sohn Ag & Co. Kg | Ofev, Vargatef | United States | Idiopathic Pulmonary Fibrosis | Boehringer Ingelheim Gmbh | 2014-10-15 | Lung Neoplasms; Breast Neoplasms; Prostatic Neoplasms; Adenocarcinoma, Clear Cell; systemic sclerosis-associated interstitial lung disease; Colorectal Neoplasms; Peritoneal Neoplasms; Gliosarcoma; Hepatic Insufficiency; Astrocytoma; Genital Neoplasms, Female; Silicosis; Sarcoma; Carcinoma, Squamous Cell; Fallopian Tube Neoplasms; Appendiceal Neoplasms; Leukemia, Myeloid, Acute; Uterine Neoplasms; Endometrial Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Adenocarcinoma; Carcinoma, Endometrioid; Scleroderma, Systemic; Telangiectasia, Hereditary Hemorrhagic; Solid tumours; Rejection of lung transplantation; Carcinoma, Renal Cell; Radiation Pneumonitis; Esophageal Neoplasms; Carcinoid Tumor; Endometrial Stromal Tumors; Idiopathic Pulmonary Fibrosis; Neoplasms; Ovarian Neoplasms; Glioblastoma; Colonic Neoplasms; Small Cell Lung Carcinoma; Pulmonary Fibrosis; Lung Diseases, Interstitial; Oligodendroglioma; Multiple Myeloma; Mesothelioma; Asbestosis; Neuroendocrine Tumors | Details |
| Avapritinib | BLU-285; CS-3007 | Approved | Blueprint Medicines Corp | Ayvakyt, Ayvakit, 泰吉华 | United States | Gastrointestinal Stromal Tumors | Blueprint Medicines Corp | 2020-01-09 | Solid tumours; Hematologic Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Mastocytosis, Systemic; Central Nervous System Neoplasms; Leukemia, Mast-Cell; Sarcoma; Breast Neoplasms; Gastrointestinal Stromal Tumors; Colorectal Neoplasms; Lung Neoplasms; Melanoma; Adenocarcinoma | Details |
| Ripretinib | DCC-2618 | Approved | Deciphera | Qinlock, 擎乐 | United States | Gastrointestinal Stromal Tumors | Deciphera Pharmaceuticals Llc | 2020-05-15 | Neoplasms; Mastocytosis, Systemic; Gastrointestinal Stromal Tumors | Details |
| Sunitinib Malate | PNU-290940AD; PHA-290940AD; SCAI-003; GB-102; PNU-290940; SU-011248-L-malate salt; PHA-290940; SU-010398; SU-11248 | Approved | Pfizer Inc | 索坦, Sutent | United States | Carcinoma, Renal Cell; Gastrointestinal Stromal Tumors | Cppi Cv | 2006-01-26 | Solid tumours; Fibromatosis, Aggressive; Ovarian Neoplasms; Kidney Neoplasms; Leukemia, Myelogenous, Chronic; Head and Neck Neoplasms; Leiomyosarcoma; Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; HIV Infections; Leukemia, Myeloid, Accelerated Phase; Leukemia; Fibrosarcoma; Teratoma; Liver Neoplasms; Ependymoma; Lymphoma, T-Cell, Peripheral; Intestinal Neoplasms; Histiocytoma, Malignant Fibrous; Carcinoma, Renal Cell; Hemangioblastoma; Carcinoma, Islet Cell; Carcinoma; Pheochromocytoma; Stomach Neoplasms; Pelvic Neoplasms; Esophageal Neoplasms; Leukemia, Hairy Cell; Polycythemia Vera; Abdominal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Thoracic Neoplasms; Pancreatic neuroendocrine tumors (pNET); Carcinoma, Ovarian Epithelial; Glioblastoma; Neurofibromatoses; Small Cell Lung Carcinoma; Adenoma, Islet Cell; Carcinoma, Transitional Cell; Pancreatic Neoplasms; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Myelodysplastic Syndromes; Hodgkin Disease; Leukemia, Myelomonocytic, Chronic; Carcinoma, Papil | Details |
| Pazopanib Hydrochloride | GSK-786034; GW-786034B; SB-786034; GW-786034 | Approved | Glaxosmithkline Plc, Novartis Pharma Ag | 维全特, Armala, Votrient, Patorma | United States | Carcinoma, Renal Cell; Sarcoma | Novartis Pharma Ag | 2009-10-19 | Uterine Cervical Diseases; Fallopian Tube Neoplasms; Lung Neoplasms; Uterine Neoplasms; Choriocarcinoma; Lymphoma; Colorectal Neoplasms; Gastrointestinal Stromal Tumors; Carcinoma, Mucoepidermoid; Gliosarcoma; Genital Neoplasms, Female; Leukemia, Myeloid, Acute; Peritoneal Neoplasms; Brain Neoplasms; Urethral Neoplasms; Medullary thyroid cancer (MTC); Chondrosarcoma, Extraskeletal Myxoid; Prostatic Neoplasms; Osteosarcoma; Neuroblastoma; Sarcoma; Breast Neoplasms; Carcinoma, Hepatocellular; Uterine Cervical Neoplasms; Breast Neoplasms, Male; Neoplasm Metastasis; von Hippel-Lindau Disease; Thyroid Cancer, Papillary; Gastrointestinal Neoplasms; Paraganglioma; Endodermal Sinus Tumor; Neoplasms, Germ Cell and Embryonal; Melanoma; Gastrinoma; Macular Degeneration; Carcinoma, Non-Small-Cell Lung; Thyroid Neoplasms; Carcinoma, Squamous Cell; Carcinoma, Small Cell; Carcinoma, Embryonal; Germinoma; Glioma; Carcinoma, Neuroendocrine; Epistaxis; Carcinoma, Ovarian Epithelial; Neoplasms; Squamous Cell Carcinoma of Head a | Details |
| Lenvatinib Mesylate | MK-7902; ER-203492-00; E-7080 | Approved | Eisai Co Ltd | Kisplyx, Lenvima, Lenvima/Kisplyx, 乐卫玛 | United States | Thyroid Neoplasms | Eisai Inc | 2015-02-13 | Carcinoma, Adenoid Cystic; Paraganglioma; Melanoma; Thyroid Cancer, Papillary; Carcinoma, Hepatocellular; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Glioma; Lymphoma; Endometrial Neoplasms; Thyroid Neoplasms; Hepatic Insufficiency; Breast Neoplasms; Cholangiocarcinoma; Osteosarcoma; Solid tumours; Neuroendocrine Tumors; Adenocarcinoma, Follicular; Liver Diseases; Thyroid Carcinoma, Anaplastic; Adenocarcinoma of Lung; Kidney Diseases; Neoplasms; Pheochromocytoma; Esophageal Neoplasms; Renal Insufficiency; Carcinoma, Renal Cell; Liver Neoplasms; Ovarian Neoplasms; Biliary Tract Neoplasms | Details |
| Regorafenib | DAST; BAY-73-4506 | Approved | Bayer AG | Stivarga, Resihance | United States | Colorectal Neoplasms | Bayer Healthcare Pharmaceuticals Inc | 2012-09-27 | Fallopian Tube Neoplasms; Osteosarcoma; Sarcoma, Ewing; Colorectal Neoplasms; Gastrointestinal Stromal Tumors; Peritoneal Neoplasms; Bone Neoplasms; Bile Duct Neoplasms; Thymoma; Thyroid Neoplasms; Leukemia, Myeloid, Acute; Gastrinoma; Lung Neoplasms; Esophageal adenocarcinoma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Melanoma; Gastrointestinal Neoplasms; Somatostatinoma; Adenocarcinoma; Neoplasm Metastasis; Meningioma; Neoplasms; Solid tumours; Ovarian Neoplasms; Rectal Neoplasms; Carcinoma, Renal Cell; Hemangiosarcoma; Carcinoid Tumor; Insulinoma; Carcinoma, Islet Cell; Stomach Neoplasms; Esophageal Neoplasms; Liver Neoplasms; Carcinoma, Transitional Cell; Colonic Neoplasms; Pancreatic Neoplasms; Glioblastoma; Carcinoma, Ovarian Epithelial; Adenoma; Glucagonoma; Carcinoma, Adenoid Cystic; Sarcoma | Details |
| Olaratumab | IMC-3G3; LY-3012207 | Approved | Eli Lilly And Company | Lartruvo | United States | Sarcoma | Eli Lilly And Company | 2016-10-19 | Solid tumours; Ovarian Neoplasms; Fibrosarcoma; Leiomyosarcoma; Hemangiosarcoma; Dermatofibrosarcoma; Neoplasms; Sarcoma, Synovial; Liposarcoma; Sarcoma; Prostatic Neoplasms; Neurofibrosarcoma; Gastrointestinal Stromal Tumors; Carcinoma, Non-Small-Cell Lung; Neoplasm Metastasis | Details |
| Nintedanib Esylate | BIBF-1120 | Approved | C.H. Boehringer Sohn Ag & Co. Kg | Ofev, Vargatef | United States | Idiopathic Pulmonary Fibrosis | Boehringer Ingelheim Gmbh | 2014-10-15 | Lung Neoplasms; Breast Neoplasms; Prostatic Neoplasms; Adenocarcinoma, Clear Cell; systemic sclerosis-associated interstitial lung disease; Colorectal Neoplasms; Peritoneal Neoplasms; Gliosarcoma; Hepatic Insufficiency; Astrocytoma; Genital Neoplasms, Female; Silicosis; Sarcoma; Carcinoma, Squamous Cell; Fallopian Tube Neoplasms; Appendiceal Neoplasms; Leukemia, Myeloid, Acute; Uterine Neoplasms; Endometrial Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Adenocarcinoma; Carcinoma, Endometrioid; Scleroderma, Systemic; Telangiectasia, Hereditary Hemorrhagic; Solid tumours; Rejection of lung transplantation; Carcinoma, Renal Cell; Radiation Pneumonitis; Esophageal Neoplasms; Carcinoid Tumor; Endometrial Stromal Tumors; Idiopathic Pulmonary Fibrosis; Neoplasms; Ovarian Neoplasms; Glioblastoma; Colonic Neoplasms; Small Cell Lung Carcinoma; Pulmonary Fibrosis; Lung Diseases, Interstitial; Oligodendroglioma; Multiple Myeloma; Mesothelioma; Asbestosis; Neuroendocrine Tumors | Details |
| Avapritinib | BLU-285; CS-3007 | Approved | Blueprint Medicines Corp | Ayvakyt, Ayvakit, 泰吉华 | United States | Gastrointestinal Stromal Tumors | Blueprint Medicines Corp | 2020-01-09 | Solid tumours; Hematologic Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Mastocytosis, Systemic; Central Nervous System Neoplasms; Leukemia, Mast-Cell; Sarcoma; Breast Neoplasms; Gastrointestinal Stromal Tumors; Colorectal Neoplasms; Lung Neoplasms; Melanoma; Adenocarcinoma | Details |
| Ripretinib | DCC-2618 | Approved | Deciphera | Qinlock, 擎乐 | United States | Gastrointestinal Stromal Tumors | Deciphera Pharmaceuticals Llc | 2020-05-15 | Neoplasms; Mastocytosis, Systemic; Gastrointestinal Stromal Tumors | Details |
| Sunitinib Malate | PNU-290940AD; PHA-290940AD; SCAI-003; GB-102; PNU-290940; SU-011248-L-malate salt; PHA-290940; SU-010398; SU-11248 | Approved | Pfizer Inc | 索坦, Sutent | United States | Carcinoma, Renal Cell; Gastrointestinal Stromal Tumors | Cppi Cv | 2006-01-26 | Solid tumours; Fibromatosis, Aggressive; Ovarian Neoplasms; Kidney Neoplasms; Leukemia, Myelogenous, Chronic; Head and Neck Neoplasms; Leiomyosarcoma; Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; HIV Infections; Leukemia, Myeloid, Accelerated Phase; Leukemia; Fibrosarcoma; Teratoma; Liver Neoplasms; Ependymoma; Lymphoma, T-Cell, Peripheral; Intestinal Neoplasms; Histiocytoma, Malignant Fibrous; Carcinoma, Renal Cell; Hemangioblastoma; Carcinoma, Islet Cell; Carcinoma; Pheochromocytoma; Stomach Neoplasms; Pelvic Neoplasms; Esophageal Neoplasms; Leukemia, Hairy Cell; Polycythemia Vera; Abdominal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Thoracic Neoplasms; Pancreatic neuroendocrine tumors (pNET); Carcinoma, Ovarian Epithelial; Glioblastoma; Neurofibromatoses; Small Cell Lung Carcinoma; Adenoma, Islet Cell; Carcinoma, Transitional Cell; Pancreatic Neoplasms; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Myelodysplastic Syndromes; Hodgkin Disease; Leukemia, Myelomonocytic, Chronic; Carcinoma, Papil | Details |
| Pazopanib Hydrochloride | GSK-786034; GW-786034B; SB-786034; GW-786034 | Approved | Glaxosmithkline Plc, Novartis Pharma Ag | 维全特, Armala, Votrient, Patorma | United States | Carcinoma, Renal Cell; Sarcoma | Novartis Pharma Ag | 2009-10-19 | Uterine Cervical Diseases; Fallopian Tube Neoplasms; Lung Neoplasms; Uterine Neoplasms; Choriocarcinoma; Lymphoma; Colorectal Neoplasms; Gastrointestinal Stromal Tumors; Carcinoma, Mucoepidermoid; Gliosarcoma; Genital Neoplasms, Female; Leukemia, Myeloid, Acute; Peritoneal Neoplasms; Brain Neoplasms; Urethral Neoplasms; Medullary thyroid cancer (MTC); Chondrosarcoma, Extraskeletal Myxoid; Prostatic Neoplasms; Osteosarcoma; Neuroblastoma; Sarcoma; Breast Neoplasms; Carcinoma, Hepatocellular; Uterine Cervical Neoplasms; Breast Neoplasms, Male; Neoplasm Metastasis; von Hippel-Lindau Disease; Thyroid Cancer, Papillary; Gastrointestinal Neoplasms; Paraganglioma; Endodermal Sinus Tumor; Neoplasms, Germ Cell and Embryonal; Melanoma; Gastrinoma; Macular Degeneration; Carcinoma, Non-Small-Cell Lung; Thyroid Neoplasms; Carcinoma, Squamous Cell; Carcinoma, Small Cell; Carcinoma, Embryonal; Germinoma; Glioma; Carcinoma, Neuroendocrine; Epistaxis; Carcinoma, Ovarian Epithelial; Neoplasms; Squamous Cell Carcinoma of Head a | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Lucitanib | S-80881; AL-3810; CO-3810; E-3810; S-80881-2 | Phase 3 Clinical | Advenchen Laboratories Nanjing Ltd | Solid tumours; Stomach Neoplasms; Small Cell Lung Carcinoma; Nasopharyngeal Carcinoma; Breast Neoplasms; Colorectal Neoplasms; Genital Neoplasms, Female; Thymus Neoplasms; Lung Neoplasms; Carcinoma, Small Cell; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | Details |
| Crenolanib Besylate | CP-868596-26; IND-112201; CP-868596; ARO-002; ARO-002-26 | Phase 3 Clinical | Pfizer Inc | Gastrointestinal Stromal Tumors; Leukemia, Myeloid, Acute; Glioma | Details |
| TLX-300 | TLX300; TLX-300; TLX-300-CDx | Phase 1 Clinical | Eli Lilly And Company | Neoplasms; Soft Tissue Neoplasms; Sarcoma | Details |
| AZD-3229 | NB-003; NB003; AZD-3229; AZD3229 | Phase 1 Clinical | Astrazeneca Turkey | Solid tumours; Gastrointestinal Stromal Tumors; Influenza B virus infection | Details |
| Lucitanib | S-80881; AL-3810; CO-3810; E-3810; S-80881-2 | Phase 3 Clinical | Advenchen Laboratories Nanjing Ltd | Solid tumours; Stomach Neoplasms; Small Cell Lung Carcinoma; Nasopharyngeal Carcinoma; Breast Neoplasms; Colorectal Neoplasms; Genital Neoplasms, Female; Thymus Neoplasms; Lung Neoplasms; Carcinoma, Small Cell; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | Details |
| Crenolanib Besylate | CP-868596-26; IND-112201; CP-868596; ARO-002; ARO-002-26 | Phase 3 Clinical | Pfizer Inc | Gastrointestinal Stromal Tumors; Leukemia, Myeloid, Acute; Glioma | Details |
| TLX-300 | TLX300; TLX-300; TLX-300-CDx | Phase 1 Clinical | Eli Lilly And Company | Neoplasms; Soft Tissue Neoplasms; Sarcoma | Details |
| AZD-3229 | NB-003; NB003; AZD-3229; AZD3229 | Phase 1 Clinical | Astrazeneca Turkey | Solid tumours; Gastrointestinal Stromal Tumors; Influenza B virus infection | Details |
This web search service is supported by Google Inc.
