 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Kat. Nr. | Arten | Produktbeschreibung | Struktur | Reinheit | Merkmal |
|---|---|---|---|---|---|
| REN-M5222 | Mouse | Mouse RENIN Protein, His Tag |  |
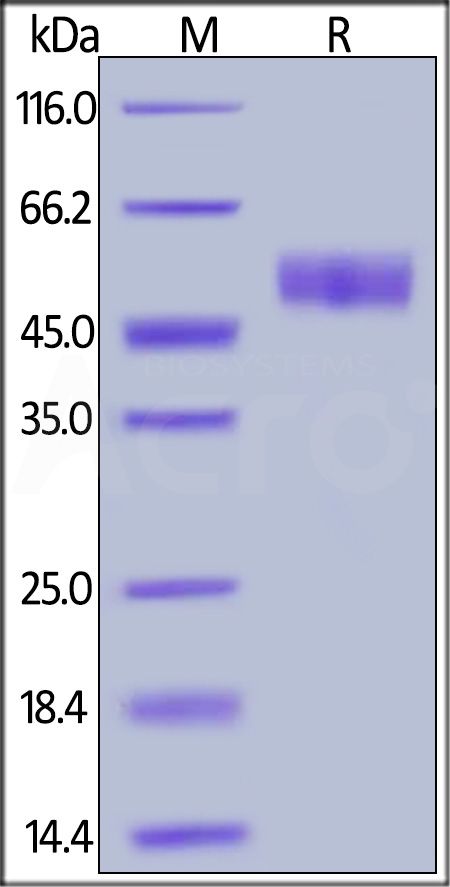
|
|
| REN-H5221 | Human | Human RENIN Protein, His Tag |  |

|
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Aliskiren Hemifumarate/Amlodipine Besilate/Hydrochlorothiazide | Approved | Novartis Pharma Ag | Rasitrio, Amturnide | United States | Hypertension | null | 2010-12-21 | Hypertension; Essential Hypertension | Details | |
| Aliskiren Hemifumarate | SPP-100; SPP-100A; SPP-100B; CGP-60536; CGP-60536B | Approved | Novartis Pharma Ag | Enviage, Rasilez, Riprazo, Sprimeo, Tekturna, 锐思力 | United States | Hypertension | Noden Pharma Dac | 2007-03-05 | Myocardial Ischemia; Heart Failure; Diabetes Mellitus, Type 2; Glomerulonephritis, IGA; Myocardial Infarction; Coronary Artery Disease; Hypertension; Hypertrophy, Left Ventricular; Diabetic macular oedema; Cardiovascular Diseases; Essential Hypertension; Diabetic Nephropathies; Kidney Failure, Chronic; Diabetes Mellitus; Overweight | Details |
| Aliskiren | Approved | Mainland China | Essential Hypertension | Novartis Pharma Ag | 2010-03-26 | Essential Hypertension | Details | |||
| Aliskiren Hemifumarate/Amlodipine Besilate/Hydrochlorothiazide | Approved | Novartis Pharma Ag | Rasitrio, Amturnide | United States | Hypertension | null | 2010-12-21 | Hypertension; Essential Hypertension | Details | |
| Aliskiren Hemifumarate | SPP-100; SPP-100A; SPP-100B; CGP-60536; CGP-60536B | Approved | Novartis Pharma Ag | Enviage, Rasilez, Riprazo, Sprimeo, Tekturna, 锐思力 | United States | Hypertension | Noden Pharma Dac | 2007-03-05 | Myocardial Ischemia; Heart Failure; Diabetes Mellitus, Type 2; Glomerulonephritis, IGA; Myocardial Infarction; Coronary Artery Disease; Hypertension; Hypertrophy, Left Ventricular; Diabetic macular oedema; Cardiovascular Diseases; Essential Hypertension; Diabetic Nephropathies; Kidney Failure, Chronic; Diabetes Mellitus; Overweight | Details |
| Aliskiren | Approved | Mainland China | Essential Hypertension | Novartis Pharma Ag | 2010-03-26 | Essential Hypertension | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| MT-2765 | MT-2765 | Phase 3 Clinical | Mitsubishi Tanabe Pharma Corp | Hypertension | Details |
| SP-20104 (Sarfez Pharmaceuticals) | SP-20104 | Phase 2 Clinical | 1 A Pharma Gmbh | Glomerulosclerosis, Focal Segmental | Details |
| Imarikiren hydrochloride | TAK-272; SCO-272 | Phase 2 Clinical | Takeda Pharmaceutical Co Ltd | Kidney Diseases; Hypertension; Diabetic Nephropathies; Hepatic Insufficiency | Details |
| MT-2765 | MT-2765 | Phase 3 Clinical | Mitsubishi Tanabe Pharma Corp | Hypertension | Details |
| SP-20104 (Sarfez Pharmaceuticals) | SP-20104 | Phase 2 Clinical | 1 A Pharma Gmbh | Glomerulosclerosis, Focal Segmental | Details |
| Imarikiren hydrochloride | TAK-272; SCO-272 | Phase 2 Clinical | Takeda Pharmaceutical Co Ltd | Kidney Diseases; Hypertension; Diabetic Nephropathies; Hepatic Insufficiency | Details |
This web search service is supported by Google Inc.
