 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Project Name | Project Stage | Molecule Type | Host Species | Therapeutic Area | Indications |
| FRα BsAb - 01 | PCC | Solid Tumor | Ovarian cancer,Non-small cell lung cancer |
| Project Name | Modality | Therapeutic Area | Indications | Stage | Right Available |
| FRα BsAb-ADC | Bispecific antibody | Oncology/Cancer | Ovarian cancer | Preclinical | Global |
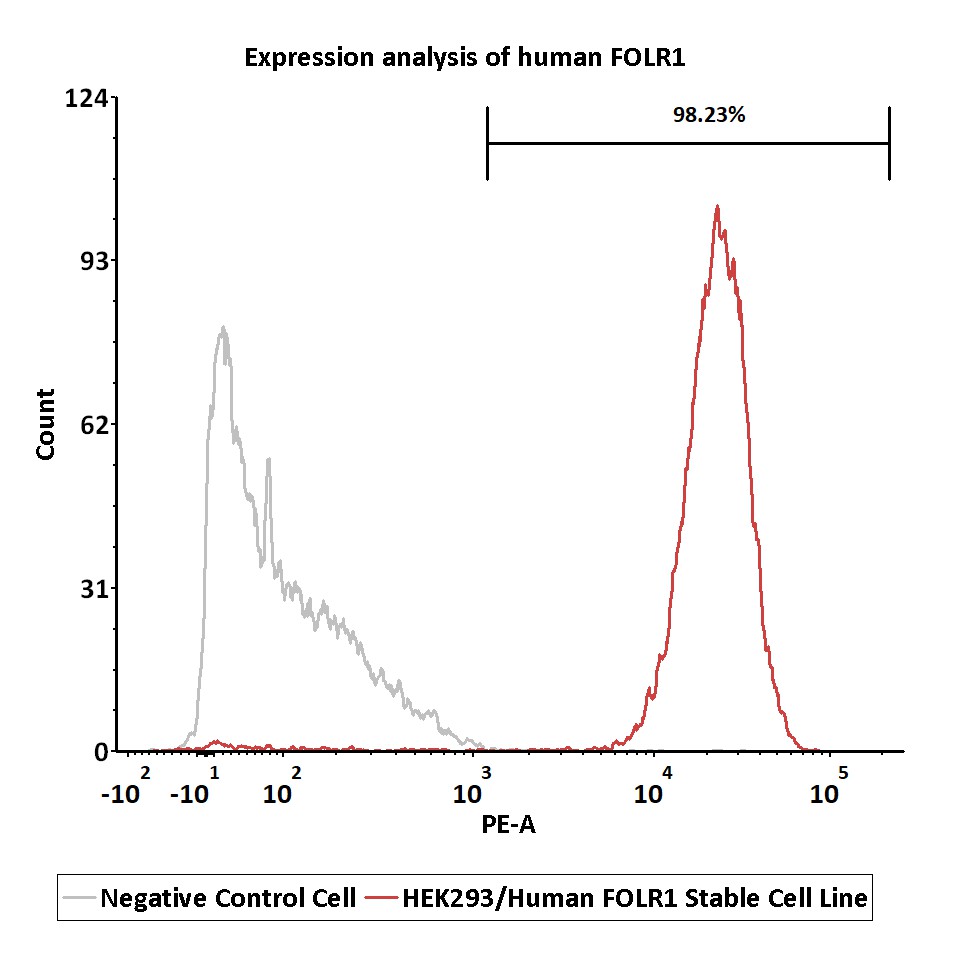
Expression analysis of human FOLR1 on HEK293/Human FOLR1 Stable Cell Line by FACS.
Cell surface staining was performed on HEK293/Human FOLR1 Stable Cell Line or negative control cell using PE-labeled anti-human FOLR1 antibody.

5e5 of anti-FOLR1 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human FOLR1, His Tag (Cat. No. FO1-HP2H9) and negative control protein respectively. PE signal was used to evaluate the binding activity (QC tested).

Biotinylated Human FOLR1, His,Avitag (Cat. No. FO1-H82E2) immobilized on SA Chip can bind Folic acid-BSA with an affinity constant of 83.8 pM as determined in a SPR assay (Biacore 8K) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Pafolacianine Sodium | Pteroyl-L-Tyr-S0456; OTL-38; EC-17; OTL-0038 | Approved | On Target Laboratories Inc | Cytalux | United States | Ovarian Neoplasms | On Target Laboratories Inc | 2021-11-29 | Ovarian Neoplasms; Inflammatory Bowel Diseases; Arthritis, Rheumatoid; Small Cell Lung Carcinoma; Neoplasms; Breast Neoplasms; Pituitary Neoplasms; Lung Neoplasms | Details |
| Mirvetuximab soravtansine | M9346-Asulfo-SPDB-DM4; IMGN-853; M9346A-sSPDB-DM4 | Approved | Immunogen Inc | Elahere | United States | Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Ovarian Epithelial | Immunogen Inc | 2022-11-14 | Ovarian Neoplasms; Solid tumours; Cystadenoma, Serous; Neoplasms; Carcinoma, Ovarian Epithelial; Triple Negative Breast Neoplasms; Breast Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Endometrial Neoplasms; Carcinosarcoma; Uterine Neoplasms; Adenocarcinoma | Details |
| Folinic Acid | FTHF | Approved | Autism Spectrum Disorder; Down Syndrome; Rectal Neoplasms; Anemia; Diarrhea; Vitamin B 12 Deficiency; Colorectal Neoplasms | Details | ||||||
| Pafolacianine Sodium | Pteroyl-L-Tyr-S0456; OTL-38; EC-17; OTL-0038 | Approved | On Target Laboratories Inc | Cytalux | United States | Ovarian Neoplasms | On Target Laboratories Inc | 2021-11-29 | Ovarian Neoplasms; Inflammatory Bowel Diseases; Arthritis, Rheumatoid; Small Cell Lung Carcinoma; Neoplasms; Breast Neoplasms; Pituitary Neoplasms; Lung Neoplasms | Details |
| Mirvetuximab soravtansine | M9346-Asulfo-SPDB-DM4; IMGN-853; M9346A-sSPDB-DM4 | Approved | Immunogen Inc | Elahere | United States | Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Ovarian Epithelial | Immunogen Inc | 2022-11-14 | Ovarian Neoplasms; Solid tumours; Cystadenoma, Serous; Neoplasms; Carcinoma, Ovarian Epithelial; Triple Negative Breast Neoplasms; Breast Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Endometrial Neoplasms; Carcinosarcoma; Uterine Neoplasms; Adenocarcinoma | Details |
| Folinic Acid | FTHF | Approved | Autism Spectrum Disorder; Down Syndrome; Rectal Neoplasms; Anemia; Diarrhea; Vitamin B 12 Deficiency; Colorectal Neoplasms | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Arfolitixorin | ISO-901; 6R-MTHF | Phase 3 Clinical | Isofol Medical Ab | Rectal Neoplasms; Colonic Neoplasms; Osteosarcoma; Colorectal Neoplasms | Details |
| Luveltamab tazevibulin | STRO-002 | Phase 3 Clinical | Sutro Biopharma Inc | Solid tumours; Ovarian Neoplasms; Carcinoma, Ovarian Epithelial; Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Endometrial Neoplasms; Carcinoma, Endometrioid | Details |
| CBP-1008 | CBP-1008 | Phase 2 Clinical | Solid tumours; Ovarian Neoplasms; Carcinoma, Ovarian Epithelial; Breast Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Squamous Cell | Details | |
| Farletuzumab | MORAb-003 | Phase 2 Clinical | Morphotek Inc | Ovarian Neoplasms; Solid tumours; Neoplasms; Carcinoma, Ovarian Epithelial; Adenocarcinoma of Lung; Peritoneal Neoplasms; Fallopian Tube Neoplasms | Details |
| Rinatabart Sesutecan | PRO-1184 | Phase 2 Clinical | ProfoundBio (Suzhou) Co Ltd | Solid tumours; Ovarian Neoplasms; Triple Negative Breast Neoplasms; Mesothelioma; Breast Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Endometrial Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| ELU-001 | ELU-001 | Phase 2 Clinical | Elucida Oncology Inc | Ovarian Neoplasms; Uterine Diseases; Carcinoma, Ovarian Epithelial; Ovarian Diseases; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Endometrial Neoplasms; Leukemia, Myeloid, Acute; Carcinoma, Endometrioid; Neoplasm Metastasis; Adenocarcinoma | Details |
| AZD5335 | AZD5335; AZD-5335 | Phase 2 Clinical | Astrazeneca Plc | Ovarian Neoplasms; Solid tumours; Adenocarcinoma of Lung | Details |
| Farletuzumab ecteribulin | MORAb-202 | Phase 2 Clinical | Morphotek Inc, Bristol-Myers Squibb Company, Eisai Co Ltd | Ovarian Neoplasms; Solid tumours; Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| BAT-8006 | BAT8006; BAT-8006 | Phase 2 Clinical | Bio-Thera Solutions Ltd | Solid tumours; Carcinoma, Ovarian Epithelial | Details |
| 4S-CAR-FRa | 4S-CAR-FRa | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Carcinoma, Transitional Cell; Urinary Bladder Neoplasms | Details |
| Noscapine-folate conjugate | Phase 1 Clinical | Emory University | Hematologic Neoplasms; Inflammation | Details | |
| MOv-18 IgE | MOv-18 IgE; MOv18; MOv-18 | Phase 1 Clinical | King'S College London, Cancer Research UK | Ovarian Neoplasms; Neoplasms | Details |
| ITIL-306 | ITIL-306 | Phase 1 Clinical | Instil Bio Inc | Solid tumours; Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Genital Neoplasms, Female; Carcinoma, Non-Small-Cell Lung | Details |
| IMGN-151 | IMGN-151 | Phase 1 Clinical | Immunogen Inc | Ovarian Neoplasms; Cystadenocarcinoma, Serous; Breast Neoplasms; Peritoneal Neoplasms; Endometrial Neoplasms; Fallopian Tube Neoplasms; Uterine Cervical Neoplasms | Details |
| AMT-151 | AMT-151 | Phase 1 Clinical | Multitude Therapeutics Inc | Solid tumours; Ovarian Neoplasms; Adenocarcinoma of Lung; Neoplasms; Triple Negative Breast Neoplasms; Carcinoma, Ovarian Epithelial; Mesothelioma; Adenocarcinoma, Clear Cell; Peritoneal Neoplasms; Carcinoma, Pancreatic Ductal; Endometrial Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Endometrioid; Adenocarcinoma | Details |
| UB-TT170 | UB-TT170; UB-TT-170; UB_TT170; UB_TT-170 | Phase 1 Clinical | Umoja BioPharma Inc | Osteosarcoma | Details |
| Arfolitixorin | ISO-901; 6R-MTHF | Phase 3 Clinical | Isofol Medical Ab | Rectal Neoplasms; Colonic Neoplasms; Osteosarcoma; Colorectal Neoplasms | Details |
| Luveltamab tazevibulin | STRO-002 | Phase 3 Clinical | Sutro Biopharma Inc | Solid tumours; Ovarian Neoplasms; Carcinoma, Ovarian Epithelial; Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Endometrial Neoplasms; Carcinoma, Endometrioid | Details |
| CBP-1008 | CBP-1008 | Phase 2 Clinical | Solid tumours; Ovarian Neoplasms; Carcinoma, Ovarian Epithelial; Breast Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Squamous Cell | Details | |
| Farletuzumab | MORAb-003 | Phase 2 Clinical | Morphotek Inc | Ovarian Neoplasms; Solid tumours; Neoplasms; Carcinoma, Ovarian Epithelial; Adenocarcinoma of Lung; Peritoneal Neoplasms; Fallopian Tube Neoplasms | Details |
| Rinatabart Sesutecan | PRO-1184 | Phase 2 Clinical | ProfoundBio (Suzhou) Co Ltd | Solid tumours; Ovarian Neoplasms; Triple Negative Breast Neoplasms; Mesothelioma; Breast Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Endometrial Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| ELU-001 | ELU-001 | Phase 2 Clinical | Elucida Oncology Inc | Ovarian Neoplasms; Uterine Diseases; Carcinoma, Ovarian Epithelial; Ovarian Diseases; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Endometrial Neoplasms; Leukemia, Myeloid, Acute; Carcinoma, Endometrioid; Neoplasm Metastasis; Adenocarcinoma | Details |
| AZD5335 | AZD5335; AZD-5335 | Phase 2 Clinical | Astrazeneca Plc | Ovarian Neoplasms; Solid tumours; Adenocarcinoma of Lung | Details |
| Farletuzumab ecteribulin | MORAb-202 | Phase 2 Clinical | Morphotek Inc, Bristol-Myers Squibb Company, Eisai Co Ltd | Ovarian Neoplasms; Solid tumours; Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| BAT-8006 | BAT8006; BAT-8006 | Phase 2 Clinical | Bio-Thera Solutions Ltd | Solid tumours; Carcinoma, Ovarian Epithelial | Details |
| 4S-CAR-FRa | 4S-CAR-FRa | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Carcinoma, Transitional Cell; Urinary Bladder Neoplasms | Details |
| Noscapine-folate conjugate | Phase 1 Clinical | Emory University | Hematologic Neoplasms; Inflammation | Details | |
| MOv-18 IgE | MOv-18 IgE; MOv18; MOv-18 | Phase 1 Clinical | King'S College London, Cancer Research UK | Ovarian Neoplasms; Neoplasms | Details |
| ITIL-306 | ITIL-306 | Phase 1 Clinical | Instil Bio Inc | Solid tumours; Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Genital Neoplasms, Female; Carcinoma, Non-Small-Cell Lung | Details |
| IMGN-151 | IMGN-151 | Phase 1 Clinical | Immunogen Inc | Ovarian Neoplasms; Cystadenocarcinoma, Serous; Breast Neoplasms; Peritoneal Neoplasms; Endometrial Neoplasms; Fallopian Tube Neoplasms; Uterine Cervical Neoplasms | Details |
| AMT-151 | AMT-151 | Phase 1 Clinical | Multitude Therapeutics Inc | Solid tumours; Ovarian Neoplasms; Adenocarcinoma of Lung; Neoplasms; Triple Negative Breast Neoplasms; Carcinoma, Ovarian Epithelial; Mesothelioma; Adenocarcinoma, Clear Cell; Peritoneal Neoplasms; Carcinoma, Pancreatic Ductal; Endometrial Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Endometrioid; Adenocarcinoma | Details |
| UB-TT170 | UB-TT170; UB-TT-170; UB_TT170; UB_TT-170 | Phase 1 Clinical | Umoja BioPharma Inc | Osteosarcoma | Details |
This web search service is supported by Google Inc.
