 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
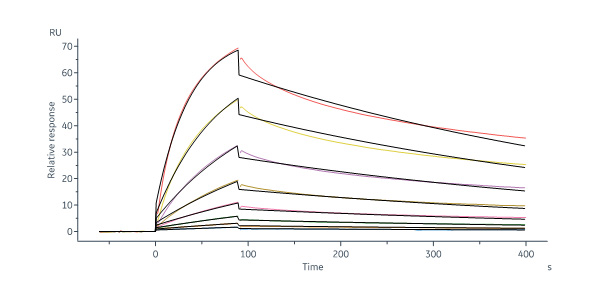
Human DLL4 Protein, Fc Tag, premium grade (Cat. No. DL4-H5255) captured on Protein A Chip can bind Human NOTCH1 Protein, His Tag, premium grade (Cat. No. NO1-H52H3) with an affinity constant between 1.00 nM - 150 nM as determined in a SPR assay (Biacore 8K) (QC tested).
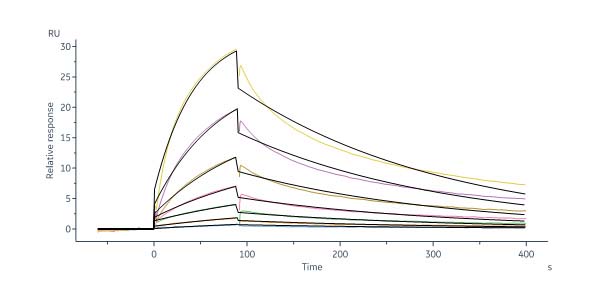
GMP Human DLL4 Protein, Fc Tag (Cat. No. GMP-DL4H28) captured on Protein A Chip can bind Human NOTCH1 Protein, His Tag, premium grade (Cat. No. NO1-H52H3) with an affinity constant between 1.00 nM - 150 nM as determined in a SPR assay (Biacore 8K) (QC tested).
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| TR-009 | NOV-1501; ABL001-ABL Bio; ABL-001-ABL Bio; ES-104; CTX-009; HD-B001A; HDB001A; TR-009 | Phase 3 Clinical | Abl Bio Inc | Biliary Tract Neoplasms; Solid tumours; Rectal Neoplasms; Colonic Neoplasms; Neoplasms; Cholangiocarcinoma; Bile Duct Neoplasms; Colorectal Neoplasms; Gallbladder Neoplasms | Details |
| Navicixizumab | OMP-305B83 | Phase 3 Clinical | Oncomed Pharmaceuticals Inc | Ovarian Neoplasms; Solid tumours; Stomach Neoplasms; Triple Negative Breast Neoplasms; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms | Details |
| Dilpacimab | ABT-165; DVD-Ig ABT-165 | Phase 1 Clinical | Abbvie Inc | Solid tumours; Neoplasms | Details |
| TR-009 | NOV-1501; ABL001-ABL Bio; ABL-001-ABL Bio; ES-104; CTX-009; HD-B001A; HDB001A; TR-009 | Phase 3 Clinical | Abl Bio Inc | Biliary Tract Neoplasms; Solid tumours; Rectal Neoplasms; Colonic Neoplasms; Neoplasms; Cholangiocarcinoma; Bile Duct Neoplasms; Colorectal Neoplasms; Gallbladder Neoplasms | Details |
| Navicixizumab | OMP-305B83 | Phase 3 Clinical | Oncomed Pharmaceuticals Inc | Ovarian Neoplasms; Solid tumours; Stomach Neoplasms; Triple Negative Breast Neoplasms; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms | Details |
| Dilpacimab | ABT-165; DVD-Ig ABT-165 | Phase 1 Clinical | Abbvie Inc | Solid tumours; Neoplasms | Details |
This web search service is supported by Google Inc.
