 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
Evaluating CAR expression is an essential step in the production of CAR-T cells. This is often done by flow cytometry,using protein L, anti-Fab antibodies, anti-idiotypic antibody or target antigens as detection antibodies. Among these common choices, target antigens are widely considered to be the best option, because it offers high specificity and minimal background staining.
Click to download the Guidance for CAR detection
| Reagents | Mechanism | Pros | Cons |
|---|---|---|---|
| Target Antigens |
|
|
|
| Protein L |
|
|
|
| Anti-Fab antibody |
|
|
|
| Anti-idiotypic Antibody |
|
|
|
Please feel free to contact us by cart@acrobiosystems.com if you have any questions.

Reagents:Biotinylated human BCMA protein, Fc & Avi Tag (ACROBiosystems, Cat. No. BC7-H82F0); PE Streptavidin (Biolegend, Cat. No. 405204).
Samples:Jurkat cells expressing BCMA-CAR ; Primary T cells expressing BCMA-CAR
Brief Protocol:
Results:The data show that BCMA-CAR expression can be successfully quantified using Biotinylated BCMA from ACROBiosystems (Cat. No. BC7-H82F0) both in jurkat cell line (Fig.1) and primary T cells (Fig.2). Control cells lacking BCMA-CAR expression showed no positive staining (Fig. 3) with PE- Streptavidin conjugate indicating that the staining using biotinylated BCMA is specific.
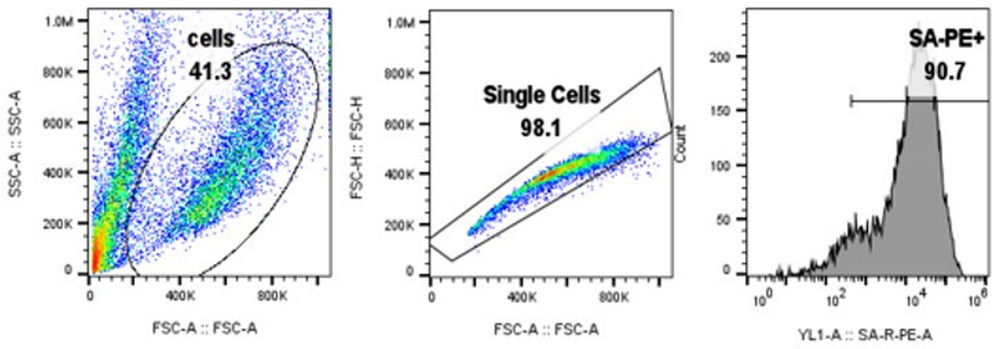 Fig. 1. BCMA-CAR expression in Jurkat cells
Fig. 1. BCMA-CAR expression in Jurkat cells
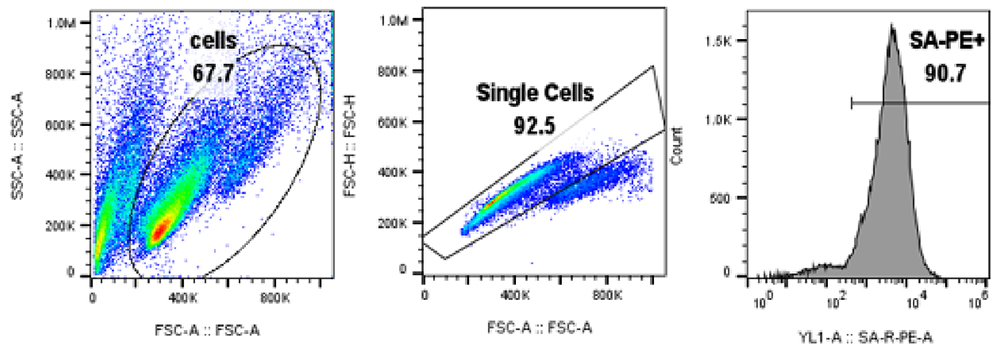 Fig. 2. BCMA-CAR expression in Primary T cells
Fig. 2. BCMA-CAR expression in Primary T cells
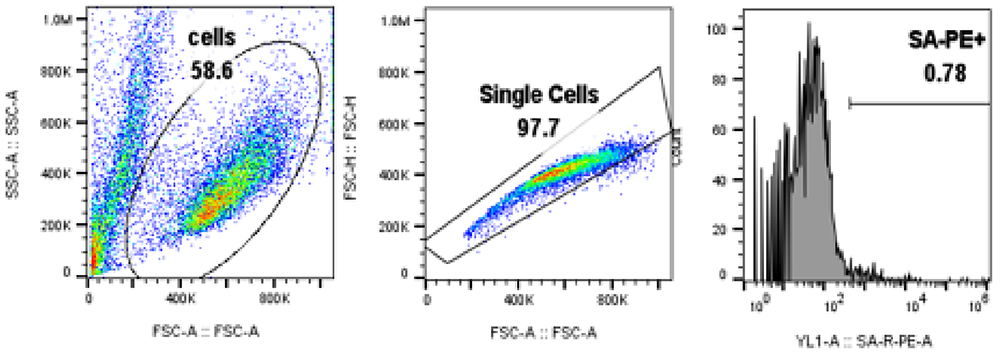 Fig. 3 Control cells without BCMA-CAR expression.
Fig. 3 Control cells without BCMA-CAR expression.
Data are kindly provided by R&D team, Theragent Inc 

Reagents:Biotinylated human BCMA protein, Fc & Avi Tag (ACROBiosystems, Cat. No. BC7-H82F0);PE Streptavidin (Biolegend, Cat. No. 405204).
Samples:Anti-BCMA CAR-transduced human primary T-cells.
Brief Protocol:
Results:The data showed that the expression of anti-BCMA CARs on transduced T cell surface from donor 1 and donor 2 were 52.72% and 73.49%, respectively.
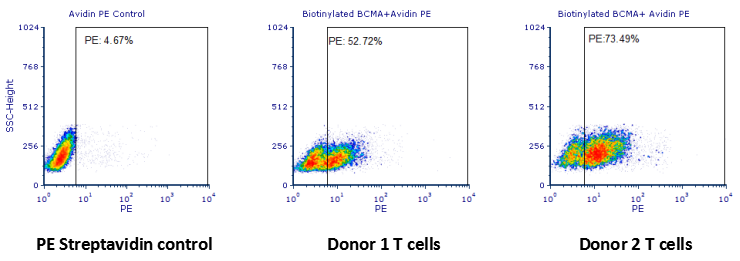 Human T cells were transfected with anti-BCMA CAR and cultured for 3 days. Three days post-transfection, 1x106 cells were first incubated with 50ul biotinylated human BCMA protein (Cat. No. BC7-H82F0, 8ug/ml ), washed and then stained with PE Streptavidin and analyzed by flow cytometry.
Human T cells were transfected with anti-BCMA CAR and cultured for 3 days. Three days post-transfection, 1x106 cells were first incubated with 50ul biotinylated human BCMA protein (Cat. No. BC7-H82F0, 8ug/ml ), washed and then stained with PE Streptavidin and analyzed by flow cytometry. Data are kindly provided by PREGENE Biopharma
Reagents:FITC-labeled Human CD19 (20-291) Protein (ACROBiosystems, Cat. No. CD9-HF2H2).
Samples:Anti-CD19 CAR-293 cells.
Brief Protocol:
Results:The data showed that the expression level of anti-CD19 scFv on the surface of anti-CD19 CAR-293 cells was 95.37%.
 293 cells were transfected with anti-CD19-scFv and RFP tag. 2e5 of the cells were stained with B. FITC-Labeled Human CD19 (20-291) (Cat. No. CD9-HF2H2, 10 µg/ml) and C. FITC-labeled protein control. A. Non-transfected 293 cells and C. FITC-labeled protein control were used as negative control. RFP was used to evaluate CAR (anti-CD19-scFv) expression and FITC was used to evaluate the binding activity of FITC-labeled Human CD19 (20-291) (Cat. No. CD9-HF2H2).
293 cells were transfected with anti-CD19-scFv and RFP tag. 2e5 of the cells were stained with B. FITC-Labeled Human CD19 (20-291) (Cat. No. CD9-HF2H2, 10 µg/ml) and C. FITC-labeled protein control. A. Non-transfected 293 cells and C. FITC-labeled protein control were used as negative control. RFP was used to evaluate CAR (anti-CD19-scFv) expression and FITC was used to evaluate the binding activity of FITC-labeled Human CD19 (20-291) (Cat. No. CD9-HF2H2).
Reagents:FITC-Labeled Monoclonal Anti-FMC63 scFv Antibody, Mouse IgG1 (ACROBiosystems, Cat. No. FM3-FY45).
Samples:Anti-CD19 CAR-293 cells.
Brief Protocol:
Results:The data showed that the expression level of anti-CD19 scFv on the surface of anti-CD19 CAR-293 cells was 100%.
 2e5 of Anti-CD19 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) FITC-Labeled Monoclonal Anti-FMC63 scFv Antibody, Mouse IgG1 (Cat. No. FM3-FY45) and isotype control respectively. FITC signal was used to evaluate the binding activity .
2e5 of Anti-CD19 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) FITC-Labeled Monoclonal Anti-FMC63 scFv Antibody, Mouse IgG1 (Cat. No. FM3-FY45) and isotype control respectively. FITC signal was used to evaluate the binding activity .
Reagents:PE-labeled Human Mesothelin / MSLN (296-580) Protein (ACROBiosystems, Cat. No. MSN-HP2H5).
Samples:Anti-MSLN CAR-293 cells
Brief Protocol:
Results:The data showed that the expression level of anti-MSLN scFv on the surface of anti-MSLN CAR-293 cells was 100 %.
 1e6 of the anti-MSLN CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human Mesothelin (296-580) Protein, His Tag (Cat. No. MSN-HP2H5) and negative control protein respectively, PE signal was used to evaluate the binding activity.
1e6 of the anti-MSLN CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human Mesothelin (296-580) Protein, His Tag (Cat. No. MSN-HP2H5) and negative control protein respectively, PE signal was used to evaluate the binding activity.
Reagents:Human CD19 Protein, Fc Tag (ACROBiosystems, Cat. No. CD9-H5259);Human CD19 Protein ,Fc Tag (Company N);Human PD-L1 Protein, Fc Tag (ACROBiosystems, Cat. No. PD1-H5258), as negative control;FITC anti-human IgG Fc antibody (Biolegend, Cat. No. 409310).
Cells:R1013-C6 cells (Transfected 293 cells expressing the anti-CD19 [FCM63] scFv & RFP tag); Expi 293 cells; Jurkat E6.1 cells.
Brief Protocol:
Results:The data shows that Company N’s human CD19-Fc fusion protein exhibits strong non-specific binding to 293 and Jurkat cells.
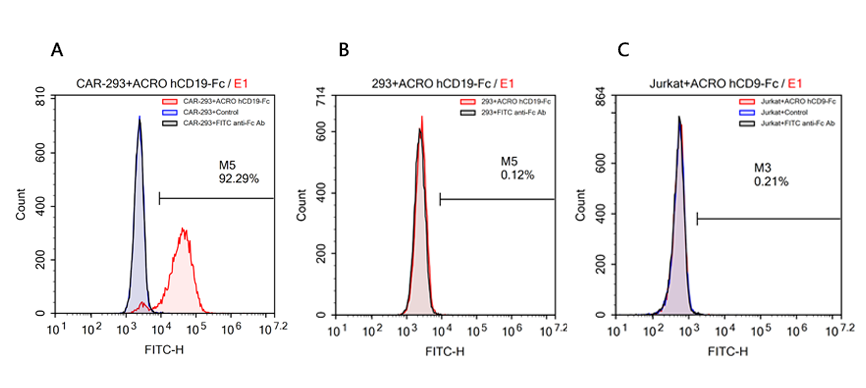 FACS analysis of human CD19 protein, Fc Tag (ACROBiosystems, Cat. No. CD9-H5259) binding to A. R1013-C6 cells, B. Expi 293 cells, C. Jurkat E6.1 cells. Cells were first stained with human CD19 protein, Fc Tag (ACROBiosystems, Cat. No. CD9-H5259) followed by FITC anti-human IgG Fc antibody, and then analyzed using NovoCyteTM Flow Cytometer. The data were analyzed with FCS Express 6Plus and GraphPad Prism 5 software.
FACS analysis of human CD19 protein, Fc Tag (ACROBiosystems, Cat. No. CD9-H5259) binding to A. R1013-C6 cells, B. Expi 293 cells, C. Jurkat E6.1 cells. Cells were first stained with human CD19 protein, Fc Tag (ACROBiosystems, Cat. No. CD9-H5259) followed by FITC anti-human IgG Fc antibody, and then analyzed using NovoCyteTM Flow Cytometer. The data were analyzed with FCS Express 6Plus and GraphPad Prism 5 software.
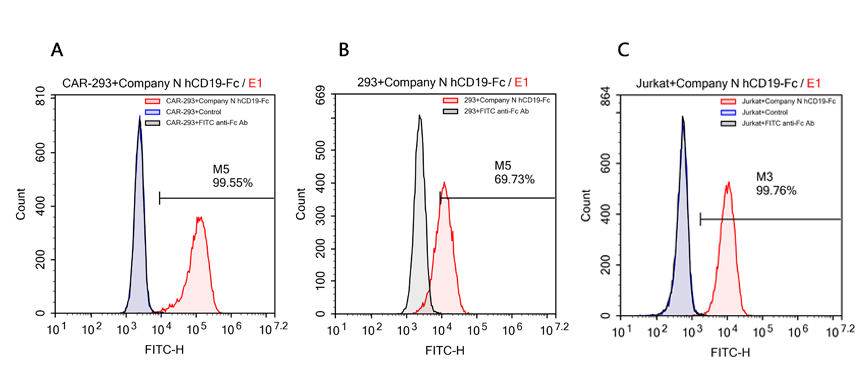 FACS analysis of human CD19 protein, Fc Tag (Company N) binding to A. R1013-C6 cells, B. Expi 293 cells, C. Jurkat E6.1 cells. Cells were first stained with human CD19 protein, Fc Tag (Company N) followed by FITC anti-human IgG Fc antibody, and then analyzed using NovoCyteTM Flow Cytometer. The data were analyzed with FCS Express 6Plus and GraphPad Prism 5 software.
FACS analysis of human CD19 protein, Fc Tag (Company N) binding to A. R1013-C6 cells, B. Expi 293 cells, C. Jurkat E6.1 cells. Cells were first stained with human CD19 protein, Fc Tag (Company N) followed by FITC anti-human IgG Fc antibody, and then analyzed using NovoCyteTM Flow Cytometer. The data were analyzed with FCS Express 6Plus and GraphPad Prism 5 software.
This web search service is supported by Google Inc.
